Question: The data from Problem P9.20 can be expressed in terms of the molality rather than the mole fraction of Br2 . Use the data from
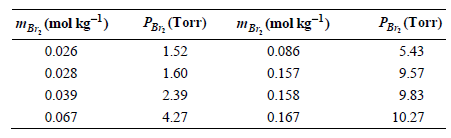
, (mol kg-l) 0.026 0.028 0.039 , (Torr) , (Torr) MBr, (mol kg-) 1.52 0.086 0.157 0.158 5.43 9.57 1.60 2.39 9.83 10.27 4.27 0.067 0.167
Step by Step Solution
3.39 Rating (171 Votes )
There are 3 Steps involved in it
The best fit line in the plot is PBr ... View full answer

Get step-by-step solutions from verified subject matter experts


