The following data show how the standard molar constant-pressure heat capacity of sulfur dioxide varies with temperature.
Question:
The following data show how the standard molar constant-pressure heat capacity of sulfur dioxide varies with temperature. By how much does the standard molar enthalpy of SO2(g) increase when the temperature is raised from 298.15K to 1500K?
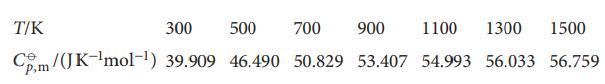
Transcribed Image Text:
T/K 300 500 700 900 1100 1300 1500 Cm/(JK-¹mol-¹) 39.909 46.490 50.829 53.407 54.993 56.033 56.759
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 84% (13 reviews)
Answer and thorough explanation The standard molar enthalpy of a substance is given by H Hf CpT Tf w...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:
Students also viewed these Sciences questions
-
The following data show how the marginal external benefit and marginal private benefit associated with a soil treatment agent to control Japanese beetles vary with the gallons of the control agent...
-
The following data show the exam grades for two different sections of a statistics class that I teach. The first group of data is from a day section of traditional students. The second group of data...
-
The following data show the daily closing prices (in dollars per share) for IBM for November 3, 2005, through December 1, 2005 (Compustat, February 26, 2006). a. Define the independent variable...
-
As manager of a local pizza parlor, you want to develop a balanced scorecard so you can more effectively monitor the restaurants performance. Required a. Propose at least two goals for each...
-
Using the information from BE14- 24, prepare the journal entry to record the bond issue, assuming that Lee Equipment Company is an IFRS reporter. BE14-24 Lee Equipment Company issued 200 eight- year,...
-
Design the values of K 1 and K 2 in the system of Figure P7.15 to meet the following specifications: Steady-state error component due to a unit step disturbance is -0.00001; steady-state error...
-
1. To develop an understanding of your ethical leadership style 2. To understand how your preferred ethical leadership style relates to other ethical leadership styles Directions 1. Please read the...
-
Reizenstein Technologies (RT) has just developed a solar panel capable of generating 200% more electricity than any solar panel currently on the market. As a result, RT is expected to experience a...
-
Corporate taxation in South Africa is too high and has negative implications for economic growth and our competitiveness with our main trading partners." Do you agree with this statement? Why (not)?...
-
The Dorilane Company specializes in producing a set of wood patio furniture consisting of a table and four chairs. The set enjoys great popularity, and the company has ample orders to keep production...
-
Describe two calorimetric methods for the determination of enthalpy changes that accompany chemical processes.
-
A sample consisting of 1.00mol Ar is expanded isothermally at 20 C from 10.0dm 3 to 30.0dm 3 (i) Reversibly, (ii) Against a constant external pressure equal to the final pressure of the gas, (iii)...
-
A department store is considering a new credit policy to try to reduce the number of customers de-faulting on payments. A suggestion is made to discontinue credit to any customer who has been one...
-
. Calculate the total excavation and embankment volumes between stations 1 and 3. (30p) S S S 0+034 Su S-14 m S21-7 m S-1B m Siz 1C m S22-6 m S ID m 0+05B 3 0+07D S S
-
A plate carries a charge of-2 C, while a rod carries a charge of 2.50 C. How many electrons must be transferred from the plate to the rod, so that both objects have the same charge? N-Number...
-
Part A A 4.00 g bullet is fired horizontally into a 1.20 kg wooden block resting on a horizontal surface. The coefficient of kinetic friction between block and surface is 0.200. The bullet remains...
-
You expect to receive $ 1 0 , 0 0 0 at the end of year 1 , $ 5 , 0 0 0 at the end of year 2 and $ 4 , 0 0 0 at the end of year 3 . If your required rate of return is 8 % , what is the uneven cash...
-
A 10 g bullet is fired into a 1 kg block of wood, where it lodges. The block then slides 4 m across a wood floor before coming to a stop (use k = 0.2 for wood on wood). A) What was the bullet's...
-
NASA is planning many Mars missions with rover vehicles. A typical rover is a solar-powered vehicle which will see where it is going with TV cameras and will measure distance to objects with laser...
-
Some people argue that the internal control requirements of the Sarbanes-Oxley Act (SOX) put U.S. companies at a competitive disadvantage to companies outside the United States. Discuss the...
-
Calculate the percentage difference in the fundamental vibration wavenumber of 23 Na 35 Cl and 23 Na 37 Cl on the assumption that their force constants are the same.
-
At low resolution, the strongest absorption band in the infrared absorption spectrum of 12 C 16 O is centred at 2150 cm 1 . Upon closer examination at higher resolution, this band is observed to be...
-
An object of mass 1.0 kg suspended from the end of a rubber band has a vibrational frequency of 2.0 Hz. Calculate the force constant of the rubber band.
-
You expect to receive $30,000 at graduation in two years. You plan on investing it at 7 percent until you have $100,000. How long will you wait from now? (Do not round intermediate calculations and...
-
A construction company recognizes revenue from construction contracts over time using the input method based on costs incurred. It reports the following: Construction costs Year 1 Year 2 $100 $200...
-
When Olga Shapiro mentioned that the financial statements were materially misstated, what kind of opinion was this?

Study smarter with the SolutionInn App


