The following equilibrium data have been obtained for the adsorption of nitrogen dioxide, NO 2 , on
Question:
The following equilibrium data have been obtained for the adsorption of nitrogen dioxide, NO2, on silica gel at 25°C and 1 atm:
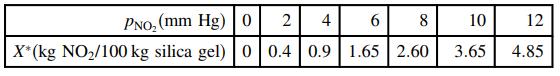
(a) Confirm that these data are reasonably correlated by the Freundlich isotherm
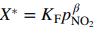
and determine the values of KF and β that provide the best correlation.
(b) The adsorption column shown in the figure below has an internal diameter of 10.0 cm and a bed height of 1.00 m. The bed of silica gel has a bulk density of 0.75 kg/L. The adsorber is to remove NO2 from a stream containing 1.0 mole% NO2 and the balance air that enters the adsorber at 8.00 kg/h. The pressure and temperature throughout the column are maintained at 1 atm and 25°C. Past experience with this system has shown that a plot of the partial-pressure ratio [(pNO2)outlet/(pNO2)inlet] versus time produces a breakthrough curve with the following appearance.
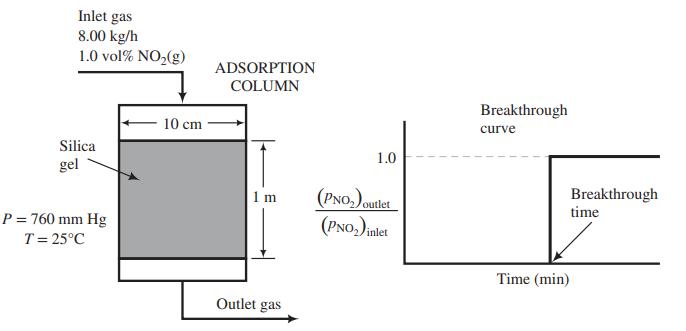
Using the isotherm derived in Part (a), determine the time (in min) required for breakthrough of the NO2.
(c) Silica gel in the column can be regenerated (i.e., adsorbed NO2 can be removed so that the silica gel column can be reused) by elevating the bed temperature and/or purging the bed with clean air. Suppose such a regeneration process requires 1.5 hours to accomplish. Process shutdowns can be avoided by installing several silica gel columns in parallel, using one to carry out the purification while the others are being regenerated. What is the minimum number of columns required to achieve continuous process operation?
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard




