The gas-phase reaction between methanol and acetic acid to form methyl acetate and water takes place in
Question:
The gas-phase reaction between methanol and acetic acid to form methyl acetate and water
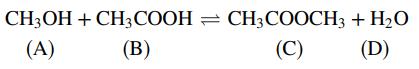
takes place in a batch reactor. When the reaction mixture comes to equilibrium, the mole fractions of the four reactive species are related by the reaction equilibrium constant
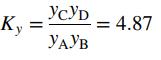
(a) Suppose the feed to the reactor consists of nA0; nB0; nC0; nD0, and nI0 gram-moles of A, B, C, D, and an inert gas, I, respectively. Let ξ be the extent of reaction. Write expressions for the gram-moles of each reactive species in the final product, nA(ξ); nB(ξ); nC(ξ), and nD(ξ). Then use these expressions and the given equilibrium constant to derive an equation for ξe , the equilibrium extent of reaction, in terms of nA0; ... ; nI0.
(b) If the feed to the reactor contains equimolar quantities of methanol and acetic acid and no other species, calculate the equilibrium fractional conversion.
(c) It is desired to produce 70 mol of methyl acetate starting with 75 mol of methanol. If the reaction proceeds to equilibrium, how much acetic acid must be fed? What is the composition of the final product?
(d) Suppose it is important to reduce the concentration of methanol by making its conversion at equilibrium as high as possible, say 99%. Again assuming the feed to the reactor contains only methanol and acetic acid and that it is desired to produce 70 mol of methyl acetate, determine the extent of reaction and quantities of methanol and acetic acid that must be fed to the reactor.
(e) If you wanted to carry out the process of Part (b) or (c) commercially, what would you need to know besides the equilibrium composition to determine whether the process would be profitable? (List several things.)
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





