Question: The parameter a in the van der Waals equation is greater for H 2 O than for He. What does this say about the difference
Figure 1.10
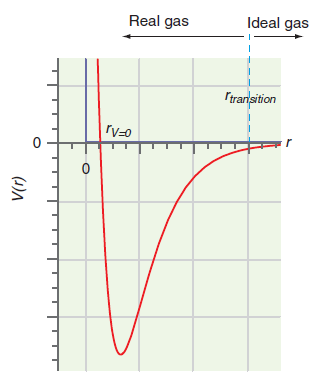
Ideal gas Real gas Itransition V=0 (1)A
Step by Step Solution
3.44 Rating (173 Votes )
There are 3 Steps involved in it
It says that the ... View full answer

Get step-by-step solutions from verified subject matter experts


