The standard potential of the AgCl/Ag,Cl couple has been measured very carefully over a range of
Question:
The standard potential of the AgCl/Ag,Cl− couple has been measured very carefully over a range of temperature (R.G. Bates and V.E. Bowers, J. Res. Nat. Bur. Stand. 53, 283 (1954)) and the results were found to fit the expression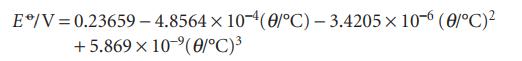
Calculate the standard Gibbs energy and enthalpy of formation of Cl−(aq) and its entropy at 298 K.
Transcribed Image Text:
E/V=0.23659-4.8564 × 10-4 (0/°C) -3.4205 x 10-6 (0/°C)² +5.869 x 10 ⁹(0/°C)³
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
Standard potential of the AgClAgCl couple as per the fit expression E o V 023659 48564 x 10 4 x 298 ...View the full answer

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The standard potential of the Zn2+/ Zn electrode is -0.76 V at 25e. The exchange current density for H+ discharge at platinum is 0.79 mA cm-2 Can zinc be plated on to platinum at that temperature?...
-
The standard enthalpy of formation of H2O(l) at 298 K is 285.8 kJ/ mol. Calculate the change in internal energy for the following process at 298 K and 1 atm: H2O(l) H2(g) + O2(g) Eo = ?
-
At 298 K the standard enthalpy of combustion of sucrose is -5797 k] mol-I and the standard Gibbs energy of the reaction is -6333 k] mol ". Estimate the additional non-expansion work that may be...
-
A rectangular loop of wire 24 cm by 72 cm is bent into an L shape, as shown in FIGURE 23-49. The magnetic field in the vicinity of the loop has a magnitude of 0.035 T and points in a direction...
-
Consider the following set of observations: You should not assume these data come from a normal distribution. Test the hypothesis that these data come from a distribution with a median equal to 4....
-
A square aluminum plate 5 mm thick and 200 mm on a side is heated while vertically suspended in quiescent air at 40C. Determine the average heat transfer coefficient for the plate when its...
-
Internal auditors are often used to review an organizations financial statements such as balance sheets, income statements, and cash flow statements prior to public filings. Auditors seek to verify...
-
For its three investment centers, Paige Company accumulates the following data: Compute the return on investment (ROI) for eachcenter. III II Sales Controllable margin Average operating assets $...
-
Compute the missing amount in the accounting equation for each entity from the financial information presented: Assets Liabilities Equity Your Basket $ ? $ 28,000 46,000 Flowers and Gifts 85,000 ?...
-
Why was the Philly311 project so successful? What management, organization, and technology factors contributed to its success?
-
The emf of the cell Ag|AgI(s)|AgI(aq)|Ag is +0.9509 V at 25C. Calculate (a) The solubility product of AgI and (b) Its solubility.
-
If the mitochondrial electric potential between matrix and the intermembrane space were 70 mV, as is common for other membranes, how much ATP could be synthesized from the transport of 4 mol H + ,...
-
Find the sum of all the integers between 100 and 400 that are multiples of 6.
-
How do team-based incentive structures, performance feedback mechanisms, and recognition systems influence team motivation, cohesion, and accountability, and how can organizations design these...
-
One of the factors that determines the success of a social media campaign is selecting the right channels. Discuss how the social media channels (Instagram and TikTok) can help one reach their target...
-
investigate a social media public relations (PR) campaign by an organization. Some examples of platforms are LinkedIn, Twitter, Facebook, Google+, Pinterest, and YouTube. The organizations can be...
-
How can you Improve the tone and clarity of a message by using positive and courteous expressions, bias-free language, plain words, and precise terms.?
-
How do high-performance teams navigate the tension between task cohesion and social cohesion to achieve optimal levels of productivity, innovation, and interpersonal harmony ?
-
General Wireless has just completed market research on a new smart phone. This new phone is lighter and smaller, though more feature rich, than its existing product. New product sales are estimated...
-
The bookkeeper for Riley, Inc., made the following errors: a. A cash purchase of supplies of $357 was recorded as a debit to Supplies for $375 and a credit to Cash of $375. b. A cash sale of $3,154...
-
In a double-glazed window, the panes of glass are separated by 1.0 cm. What is the rate of transfer of heat by conduction from the warm room (28 C) to the cold exterior (15 C) through a window of...
-
A lump of sucrose of mass 10.0 g is suspended in the middle of a spherical flask of water of radius 10 cm at 25 C. What is the concentration of sucrose at the wall of the flask after (a) 1.0h, (b)...
-
Confirm that is a solution of the diffusion equation with convection (eqn 19C.10) with all the solute concentrated at x=x 0 at t=0 and plot the concentration profile at a series of times to show how...
-
Blackwell's is one of the largest distributors of academic books in the UK. It entered into 3 separate Sale and Purchase agreements with Toyota Motors. Each contract was for the purchase of 1 limited...
-
James "Buster" Douglas and his manager John Johnson, entered into a loxing promotion agreement on December 31, 1988 (the "Promotional Agreement" or "Agreement"), with Don King Productions, Inc....
-
Explain and employ a range of psychological concepts within neuroscience; sensation and perception; states of consciousness; development; learning; memory; motivation and emotions; health and stress;...

Study smarter with the SolutionInn App


