Question: Use mathematical software to estimate the -electron binding energy of (i) azulene (3), (ii) acenaphthalene (4) within the Hckel approximation. 3 Azulene 4 Acenaphthalene
Use mathematical software to estimate the π-electron binding energy of (i) azulene (3), (ii) acenaphthalene (4) within the Hückel approximation.
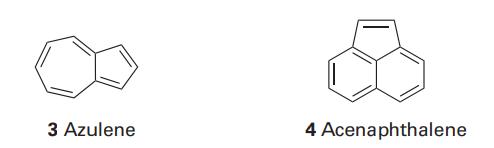
3 Azulene 4 Acenaphthalene
Step by Step Solution
3.51 Rating (161 Votes )
There are 3 Steps involved in it
Electron binding energies of closedshell molecules and ions that are large by the standards of ab in... View full answer

Get step-by-step solutions from verified subject matter experts


