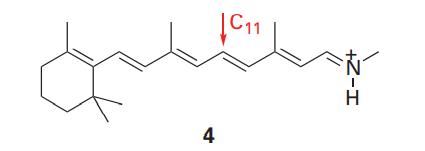
Use molecule (4) as a model of the trans conformation of the chromophore found in rhodopsin. In
Question:
Use molecule (4) as a model of the trans conformation of the chromophore found in rhodopsin. In this model, the methyl group bound to the nitrogen atom of the protonated Schiff ’s base replaces the protein.
(a) Using molecular modelling software and the computational method of your instructor’s choice, calculate the energy separation between the HOMO and LUMO of (4).
(b) Repeat the calculation for the 11-cis form of (4).
(c) Based on your results from parts (a) and (b), do you expect the experimental frequency for the π*←π visible absorption of the trans form of (4) to be higher or lower than that for the 11-cis form of (4)?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:





