Use the vapor pressures of ice given here to calculate the enthalpy of sublimation using a graphical
Question:
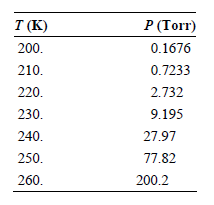
Transcribed Image Text:
T (K) P (Torr) 200. 0.1676 210. 0.7233 2.732 220. 230. 9.195 240. 27.97 250. 77.82 200.2 260.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
A least squares fit of ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the vapor pressures of SO 2 (l) given in the following table to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. T (K) 190. P (Pa) T (K) 230. P...
-
Use the vapor pressures of n-butane given in the following table to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. P (Pa) 1000 x 104 1000 x 105 T...
-
Use the vapor pressures of tetrachloromethane given in the following table to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. P/Pa T (K) 320. 330....
-
A Norman window has the outline of a semicircle on top of a rectangle. Suppose there are 8 + feet of wood trim available. Discuss why a window designer might want to maximize the area of the window....
-
Select 4 similar size public companies in the same industry. The company's size can be measured by total assets or total sales. Obtain the most recent financial statements of these companies, apply...
-
National Acceptance Company loaned Ultra Precision Industries \($692,000,\) and to secure repayment of the loan, Ultra executed a chattel mortgage security agreement on Nationals behalf on March 7,...
-
Show that, for the isotropic case, (i) the velocity correlation function is now symmetric and (ii) the pressure-velocity correlation is zero.
-
Gothic Architecture is a new chain of clothing stores specializing in the color black. Gothic issues 1,000 shares of its $1 par value common stock at $30 per share. Record the issuance of the stock....
-
What is the value of a perpetuity that pays $100 every 6 months forever? if The discount rate quoted on an APR basis is 5.6%.
-
Find all values of a and b for which A and B are both not invertible Aa+b-10 0 3 9 0 2a-36-7
-
The phase diagram of NH 3 can be characterized by the following information. The normal melting and boiling temperatures are 195.2 and 239.82 K, respectively; the triple point pressure and...
-
Calculate the vapor pressure for a mist of spherical water droplets of radius a. 1.95 10 8 m b. 2.25 10 6 m at 298 K. The vapor pressure of water at this temperature is 25.2 Torr.
-
Refer to the Applied Spectroscopy study of the optical density (y) for infrared absorption of the liquid PPFPO, Exercise 11.63. In addition to optical density, band frequency (x 1 ) and film...
-
What is the family-purpose doctrine?
-
True Or False Children who engage in activities reserved for adults are always held to the standard of care of a reasonable adult.
-
What is the doctrine of res ipsa loquitur and how does it help plaintiffs?
-
What must a plaintiff show to prove negligence per se?
-
What is the status of automobile-guest statutes today?
-
Show that the data in Table 9.6 satisfy the definitions of Gibbs and Helmholtz free energy, (1) ?G r =?H r ? T?S r and (2) ?H r = ?U r + p?V r . Quantity Value AUr Reaction internal energy +175.8...
-
Using thermodynamic data from Appendix 4, calculate G at 258C for the process: 2SO 2 (g) + O 2 (g) 88n 2SO 3 (g) where all gases are at 1.00 atm pressure. Also calculate DG8 at 258C for this same...
-
Find the gradient of the function g(x,y,z) = ax 3 + ye bz , where a and b are constants.
-
Find r where r = ix + jy + kz.
-
Find the Laplacian of the function f = exp (x 2 + y 2 + z 2 ) = e x2 e y2 e z2 .
-
The Foundational 15 (Algo) [LO1-1, LO1-2, LO1-3, LO1-4, LO1-5, LO1-6] [The following information applies to the questions displayed below.] Martinez Company's relevant range of production is 7,500...
-
Taylor Swift's most recent tour was a success by every measure. Named after the bestselling album of her career 1989, it grossed more than $250 million worldwide the top tour of 2015 raved about the...
-
Activity Purchasing material Receiving material Setting up equipment Machine depreciation and maintenance Ensuring regulatory compliance Shipping Total estimated cost Recommended Cost Driver Number...

Study smarter with the SolutionInn App


