When 1.3584 g of sodium acetate trihydrate was mixed into 100.0 cm 3 of 0.2000 m HCl(aq)
Question:
When 1.3584 g of sodium acetate trihydrate was mixed into 100.0 cm3 of 0.2000 m HCl(aq) at 25°C in a solution calorimeter, its temperature fell by 0.397°C on account of the reaction:
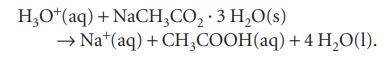
The heat capacity of the calorimeter is 91.0 J K−1 and the heat capacity density of the acid solution is 4.144 J K−1 cm−3. Determine the standard enthalpy of formation of the aqueous sodium cation. The standard enthalpy of formation of sodium acetate trihydrate is −1064 kJ mol−1.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





