Question: You are given the following data for the decomposition of acetaldehyde: Determine the order of the reaction and the rate constant for the reaction. Initial
You are given the following data for the decomposition of acetaldehyde:
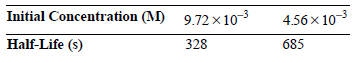
Determine the order of the reaction and the rate constant for the reaction.
Initial Concentration (M) 9.72 103 4.56x 103 Half-Life (s) 328 685
Step by Step Solution
★★★★★
3.43 Rating (159 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Since the halflife depends on the initial concentration the reaction cannot be first order To see ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


