Question: Review Conceptual Example 5 before attempting this problem. To illustrate the effect of ice on the aluminum cooling plate, consider the drawing shown here and
Review Conceptual Example 5 before attempting this problem. To illustrate the effect of ice on the aluminum cooling plate, consider the drawing shown here and the data that it contains. Ignore any limitations due to significant figures.
Conceptual Example 5
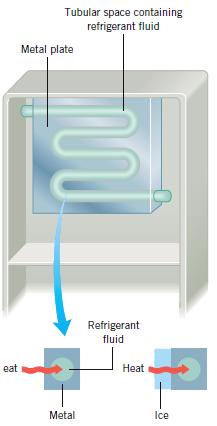
In a refrigerator, heat is removed by a cold refrigerant fluid that circulates within a tubular space embedded inside a metal plate, as Figure 13.12 illustrates. A good refrigerator cools food as quickly as possible. Which arrangement works best:
(a) An aluminum plate coated with ice,
(b) An aluminum plate without ice,
(c) A stainless steel plate coated with ice, or
(d) A stainless steel plate without ice?
Reasoning Figure 13.12 (see the blow-ups) shows the metal cooling plate with and without a layer of ice. Without ice, heat passes by conduction through the metal plate to the refrigerant fluid within. For a given temperature difference across the thickness of the metal, the rate of heat transfer depends on the thermal conductivity of the metal. When the plate becomes coated with ice, any heat that is removed by the refrigerant fluid must first be transferred by conduction through the ice before it encounters the metal plate.
Answers (a), (c), and (d) are incorrect. For answers (a) and (c), the relation Q = (kA Δ T )t/L (Equation 13.1) indicates that the heat conducted per unit time (Q/t) is inversely proportional to the thickness L of the ice. As ice builds up, the heat removed per unit time by the cooling plate decreases. Thus, when covered with ice, the cooling plate—regardless of whether it’s made from aluminum or stainless steel—does not work as well as a plate that is ice-free. Answer (d)—the stainless steel plate without ice—is incorrect, because heat is transferred more readily through a plate that has a greater thermal conductivity, and stainless steel has a smaller thermal conductivity than does aluminum (see Table 13.1).
Answer (b) is correct. The relation Q = (kAΔ T )t/L (Equation 13.1) shows that the heat conducted per unit time (Q/t) is directly proportional to the thermal conductivity k of the metal plate. Since the thermal conductivity of aluminum is more than 17 times greater than the thermal conductivity of stainless steel (see Table 13.1), aluminum is the preferred plate. The aluminum plate arrangement works best without an ice buildup. When ice builds up, the heat removed per unit time decreases because of the increased thickness of material through which the heat must pass.
(a) Calculate the heat per second per square meter that is conducted through the ice–aluminum combination.
(b) Calculate the heat per second per square meter that would be conducted through the aluminum if the ice were not present. Notice how much larger the answer is in (b) as compared to (a).
Tubular space containing refrigerant fluid Metal plate Refrigerant fluid eat Heat Metal Ice
Step by Step Solution
3.42 Rating (168 Votes )
There are 3 Steps involved in it
The energy Q conducted through a layer of material thickness L and surface area A in a time t is Q k... View full answer

Get step-by-step solutions from verified subject matter experts


