Question: In begin by drawing a diagram that shows the relations among the variables. Let U = (P, V, T ) be the internal energy of
In begin by drawing a diagram that shows the relations among the variables.
Let U = ƒ(P, V, T ) be the internal energy of a gas that obeys the ideal gas law PV = nRT (n and R constant). Find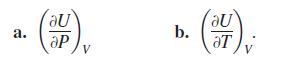
a. (SP)V b. (7)
Step by Step Solution
3.39 Rating (161 Votes )
There are 3 Steps involved in it
To find the partial derivatives of U with respect to P and T w... View full answer

Get step-by-step solutions from verified subject matter experts


