Specific heat C v is the amount of heat required to raise the temperature of one mole
Question:
Specific heat Cv is the amount of heat required to raise the temperature of one mole (gram molecule) of a gas with constant volume by 1°C. The specific heat of oxygen depends on its temperature T and satisfies the formula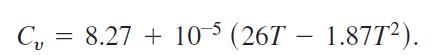
Find the average value of Cv for 20° ≤ T ≤ 675°C and the temperature at which it is attained.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thomas Calculus Early Transcendentals
ISBN: 9780321884077
13th Edition
Authors: Joel R Hass, Christopher E Heil, Maurice D Weir
Question Posted:





