A solid sample weighing 0.237 6 g contained only malonic acid and aniline hydrochloride. It required 34.02
Question:
A solid sample weighing 0.237 6 g contained only malonic acid and aniline hydrochloride. It required 34.02 mL of 0.087 71 M NaOH to neutralize the sample. Find the weight percent of each component in the solid mixture. The reactions are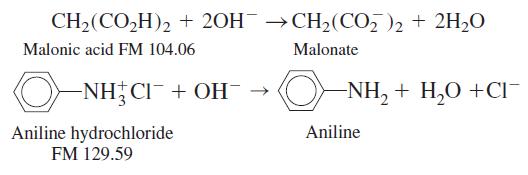
Transcribed Image Text:
CH2(CO,H)2 + 20H →CH2(CO, )2 + 2H,0 Malonic acid FM 104.06 Malonate -NH CI¯+ OH¯ → O- NH, + H,0 +CI¯ Aniline Aniline hydrochloride FM 129.59
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
3402 mL 08771 M NaOH 34020087711000 29810 3 mole of NaOH ...View the full answer

Answered By

TIRTHA MONDAL
I have completed my graduation and post graduation from Jadavpur University. I have also done Bachelor in Education (B.Ed) from the same university. I have experience in teaching JEE& NEET aspirants.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A solid mixture weighing 0.5485 g contained only ferrous ammonium sulfate hexahydrate and ferrous chloride hexahydrate. The sample was dissolved in 1M H2SO4, oxidized to Fe3+ with H2O2, and...
-
A mixture weighing 7.290 mg contained only cyclohexane, C6H12(FM 84.159), and oxirane, C2H4O (FM 44.053). When the mixture was analyzed by combustion analysis, 21.999 mg of CO2 (FM 44.010) were...
-
It takes 97.62 mL of 0.0546 M NaOH to titrate a 25.00 mL sample of H2SO4. What is the concentration of H2SO4? You will need to write the balanced chemical equation first.
-
The production of paper involves a pulping step to break down wood chips into cellulose and lignin. In the Kraft process, an aqueous, pulping-feed solution, known as white liquor, is used that...
-
Use the GSS 1OSSDS data file to study the relationship between the number of siblings a respondent has (SIBS) and his or her number of children (CHILDS). a. Construct a scatterplot of these two...
-
What are the components of the zero vector based at P = (3, 5)?
-
Two objects of inertias \(m_{1}\) and \(m_{2}\) start from rest and then interact with each other (assume neither is interacting with any other object). (a) What is the ratio of their \(x\)...
-
On January 1. Ruiz Company issued bonds as follows: Face Value: Number of Years: Stated Interest Rate: Interest payments per year 500,000 15 7% Required: 1) Calculate the bond selling price given the...
-
Tim Drinking, Inc. used a predetermined overhead allocation rate to allocate of indirect costs to the Filling Department and the Packaging Department journal entry to record the allocation of...
-
You own 1,000 shares of stock in Avondale Corporation. You will receive a $3.45 per share dividend in one year. In two years, the company will pay a liquidating dividend of $62 per share. The...
-
A solution of NaOH was standardized by gravimetric titration of a known quantity of the primary standard, potassium hydrogen phthalate: Potassium hydrogen phthalate C 8 H 5 O 4 K, FM 204.22 The NaOH...
-
A 50.0-mL sample of 0.080 0 M KSCN is titrated with 0.040 0 M Cu + . The solubility product of CuSCN is 4.8 10 -15 . At each of the following volumes of titrant, calculate pCu + , and construct a...
-
The following information is from the 2014 annual report of Weber Corporation, a company that supplies manufactured parts to the household appliance industry. Average total assets ................
-
A share of common stock just paid a dividend of $3.4. If the expected long-run growth rate for this stock is 1%, and if investors' required rate of return is 6.6%, then what is the stock price? Round...
-
+ 25. Carter borrows $20,000 to pay for college tuition. The loan is fully amortized over a three-year period with an interest rate of 8%. What is Carter's remaining balance at the end of Year Two?...
-
Identify and research a large service business in your pathway. It must be a large, US, publicly traded company on the Fortune 500 list. Don't use a company that someone has already used. Access...
-
The data for ABC Corporation for the year ended 2019 is given below: (all values are in thousands) Financial indicators Net income Values $2355.12 Sales 27,412 Total assets 25,262 Stockholder's...
-
A digital 11R felter block diagram shown in figure a) Find the trans for function H(Z) in transfer b) Find the range stable, if gain c) Realize the terms of a and b of and b to keep the filter is...
-
Light with a wavelength of 660 nm is incident on two slits and the pattern shown in the figure is viewed on a screen. Point A is directly opposite a point midway between the two slits. What is the...
-
Using the parallel-axis theorem, determine the product of inertia of the area shown with respect to the centroidal x and y axes. 6 in. 9 in. 9 in- 4.5 in. in. 4.5 in.
-
The voltage for the following cell is 0.490 V. Find Kb for the organic base RNH2. Pt(s) H2(1.00 bar) RNH2(aq, 0.10 M), RNH+3 Cl(aq, 0.050 M) ||' S.H.E.
-
The voltage of the cell shown here is - 0.246 V. The right half-cell contains the metal ion, M2+, whose standard reduction potential is - 0.266 V. Mc2+ + 2e - E° = - 0.266 V Calculate Kf for the...
-
The following cell was constructed to find the difference in Ksp between two naturally occurring forms of CaCO3(s), called calcite and aragonite.21 buffer(pH 7.00) CaCO3(s, aragonite) PbCO3(s) ...
-
If the nth partial sum of a series n-8 Sp 7 +8 00 find an and an. a1 = n = 1 an is n = 1 an = (for n 1)
-
1. Find the Maclaurin series of the function. f(x) = +6 n = 0 ) 2. Express the integral as an infinite series. (x8t - sin(St) dt, for all x 8t W n = 1
-
The Righton Corporation is taking out a loan of $38,000 at 7.8% interest (see table below). This interest rate is locked in for the life of the loan. They recognize that if/when they want to take out...

Study smarter with the SolutionInn App


