Europium is a lanthanide element found at parts per billion levels in natural waters. It can be
Question:
Europium is a lanthanide element found at parts per billion levels in natural waters. It can be measured from the intensity of orange light emitted when a solution is illuminated with ultraviolet radiation. Certain organic compounds that bind Eu(III) are required to enhance the emission. The figure shows standard addition experiments in which 10.00 mL of sample and 20.00 mL containing a large excess of organic additive were placed in 50-mL volumetric flasks. Then Eu(III) standards (0, 5.00, 10.00, or 15.00 mL) were added and the flasks were diluted to 50.0 mL with H2O. Standards added to tap water contained 0.152 ng/mL (ppb) of Eu(III), but those added to pond water were 100 times more concentrated (15.2 ng/mL).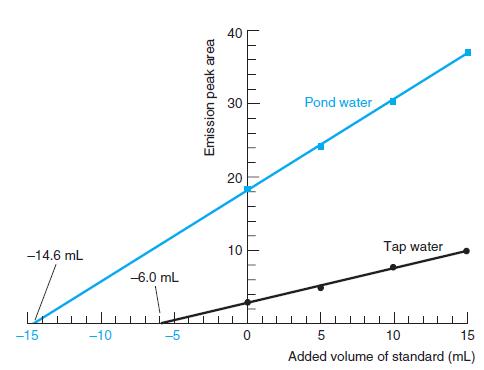
(a) Calculate the concentration of Eu(III) (ng/mL) in pond water and tap water.
(b) For tap water, emission peak area increases by 4.61 units when 10.00 mL of 0.152 ng/mL standard are added. This response is 4.61 units/1.52 ng = 3.03 units per ng of Eu(III). For pond water, the response is 12.5 units when 10.00 mL of 15.2 ng/mL standard are added, or 0.082 2 units per ng. How would you explain these observations? Why was standard addition necessary for this analysis?
Step by Step Answer:






