Internal standard graph. Data are shown below for chromatographic analysis of naphthalene (C 10 H 8 ),
Question:
Internal standard graph. Data are shown below for chromatographic analysis of naphthalene (C10H8), using deuterated naphthalene (C10D8, in which D is the isotope 2H) as an internal standard. The two compounds emerge from the column at almost identical times and are measured by a mass spectrometer.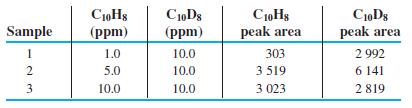
(a) Using a spreadsheet like Figure 4-15, prepare a graph of Equation 5-12 showing peak area ratio (C10H8/C10D8) versus concentration ratio ([C10H8]/[C10D8]). Find the least-squares slope and intercept and their standard uncertainties. What is the theoretical value of the intercept? Is the observed value of the intercept within experimental uncertainty of the theoretical value?
(b) Find the quotient [C10H8]/[C10D8] for an unknown whose peak area ratio (C10H8/C10D8) is 0.652. Find the standard uncertainty ux for the peak area ratio.
(c) Here is why we try not to use 3-point calibration curves. For n = 3 data points, there is n - 2 = 1 degree of freedom because 2 degrees of freedom are lost in computing the slope and intercept. Find the value of Student’s t for 95% confidence and 1 degree of freedom. From the standard uncertainty in (b), compute the 95% confidence interval for the quotient [C10H8]/[C10D8]. What is the percent relative uncertainty in the quotient [C10H8]/[C10D8]? Why do we avoid 3-point calibration curves?
Step by Step Answer:






