Lead in dry river sediment was extracted with 25 wt% HNO 3 at 358C for 1 h.
Question:
Lead in dry river sediment was extracted with 25 wt% HNO3 at 358C for 1 h. Then 1.00 mL of filtered extract was mixed with other reagents to bring the total volume to V0 5 4.60 mL. Pb(II) was measured electrochemically with a series of standard additions of 2.50 ppm Pb(II).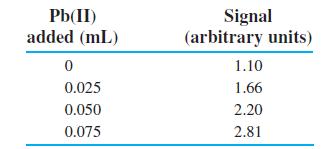
(a) Volume is not constant, so follow the procedure of Figures 5-5 and 5-6 to find ppm Pb(II) in the 1.00-mL extract.
(b) Find the standard uncertainty and 95% confidence interval for the x-intercept of the graph. Assuming that uncertainty in intercept is larger than other uncertainties, estimate the uncertainty in ppm Pb(II) in the 1.00-mL extract.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





