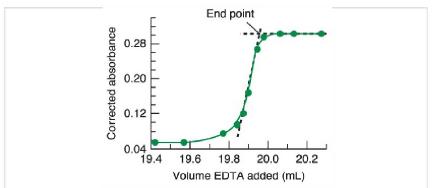
A 100-mL 100-mL sample of hard water containing magnesium and calcium was titrated as illustrated in Figure
Question:
A 100-mL 100-mL sample of hard water containing magnesium and calcium was titrated as illustrated in Figure 18-10.
It required 14.59 mL 14.59 mL of 10.83 mM 10.83 mM ethylenediaminetetraacetic acid (EDTA) to reach the end point.
a. Why does the slope of the absorbance versus volume graph change abruptly at the equivalence point?
b. How many moles of EDTA were required to reach the end point?
c. Hardness of water is the total concentration of alkaline earth ions expressed as mg CaCO3CaCO3 per liter as if all titrated cations were CaCO3CaCO3. What is the hardness of the 50-mL 50-mL sample?
Figure 18-10
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy
Question Posted:





