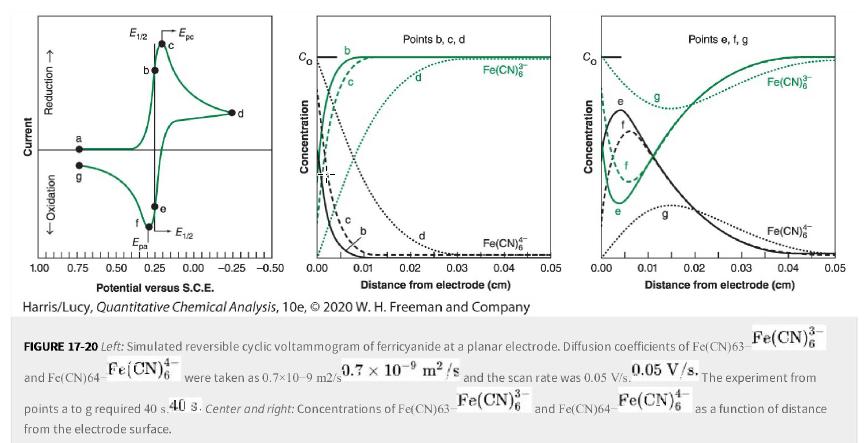
a. In Figure 17-20, is the reactant Fe(CN)63- Fe (CN) 3- 6 or Fe(CN)64-Fe(CN) 4- 6 at
Question:
a. In Figure 17-20, is the reactant Fe(CN)63- Fe(CN)3-6 or Fe(CN)64-Fe(CN)4-6 at points b, c, and d? Write the order of decreasing concentration of reactant at the electrode surface at points b, c, and d. For example, your answer might be c>b>d.c > b > d.
b. Why is there maximum current at c even though reactant concentration at the electrode is not greatest at point c? Why does current decrease from c to d even though potential is more negative at d?
c. Is the reactant Fe(CN)63- Fe(CN)3-6 or Fe(CN)64-Fe(CN)4-6 at points e, f, and g? Write the order of decreasing concentration of reactant at the electrode at points e, f, and g.
d. Why is the current magnitude greater at f than at e? Why is the current not 0 0 at g?
e. Does time of the experiment increase in the order e4-6] go through a maximum at increasing distances from the electrode at e
Figure 17-20
Step by Step Answer:

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy





