A solution containing 0.139 mmol of the triprotic acid tris(2-aminoethyl)amine 3HCl plus 0.115 mmol HCl in
Question:
A solution containing 0.139 mmol of the triprotic acid tris(2-aminoethyl)amine ∙ 3HCl plus 0.115 mmol HCl in 40 mL of 0.10 M KCl was titrated with 0.490 5 M NaOH to measure acid dissociation constants.
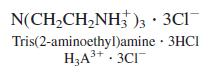
(a) Write expressions for the experimental mean fraction of protonation, n̅H(measured), and the theoretical mean fraction of protonation, n̅H(theoretical).
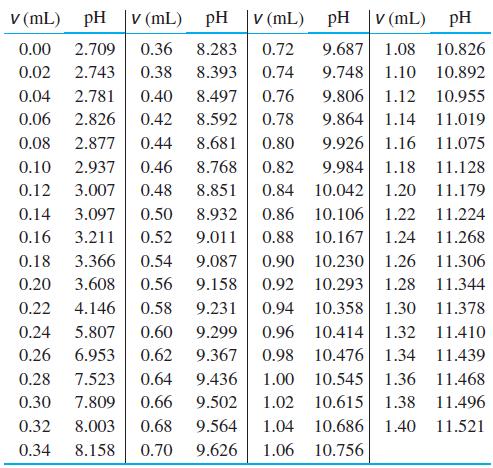
(b) From the following data, prepare a graph of n̅H(measured) versus n̅H. Find the best values of pK1, pK2, pK3, and pK'w by minimizing the sum of the squares of the residuals, Σ[n̅H (measured) – n̅H(theoretical)]2.
(c) Create a fractional composition graph showing the fractions of H3A3+, H2A2+, HA+, and A as a function of pH.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





