Cloud-point extraction. Traces of lead in drinking water cause adverse health effects. The micelle-forming surfactant Triton X-114
Question:
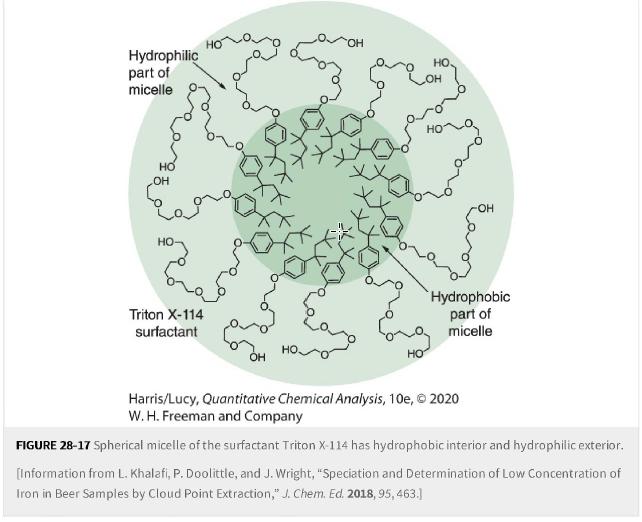
Cloud-point extraction. Traces of lead in drinking water cause adverse health effects. The micelle-forming surfactant Triton X-114 (plus the co-surfactant cetyl trimethylammonium bromide) and a crown ether ligand extract and preconcentrate Pb2+Pb2+ for analysis by inductively coupled plasma-optical emission spectrometry. The hydrophobic metal complex is extracted into the hydrophobic interior of micelles formed by Triton X-114 (Figure 28-17).
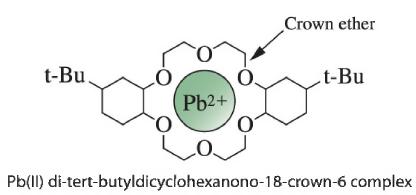
a. Surfactants and crown ether are added to 195 mL 195 mL of acidified aqueous sample. After 30 min 30 min for extraction, the solution is heated and becomes cloudy as the micelles aggregate into a separate phase containing essentially all Pb(II).Pb(II). The aqueous phase is then removed. The micellar phase is back-extracted with 5.0 mL 5.0 mL of aqueous citrate solution that complexes Pb(II) Pb(II) more tightly than the surfactant. All Pb(II) Pb(II) ends up in the aqueous phase. By what factor is Pb(II)Pb(II) preconcentrated in this procedure?
b. Drinking water with negligible lead content was fortified with 2.3 μg Pb(II)/L. 2.3 μg Pb(II)/L. Triplicate measurements yielded a mean of 1.9 μg/L 1.9 μg/L and a standard deviation of 0.2 μg/L0.2 μg/L. Find the percent recovery of the spike.
c. Does the spike recovery in (b) differ from the expected value of 2.3 μg/L Pb2+2.3 μg/L Pb2+ at the 95% 95% confidence level? If results were the same for six measurements instead of three, does the spike recovery differ from the expected value at the 95% 95% confidence level?
Figure 28-17
Step by Step Answer:

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy





