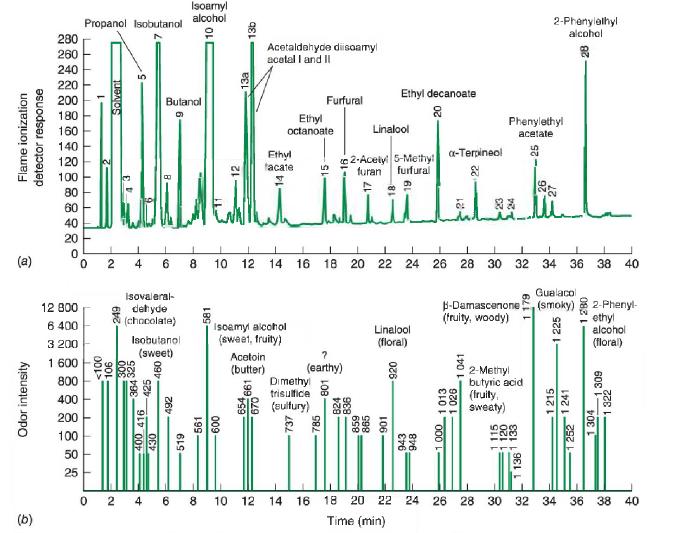
In the analysis of odorants in tequila in Figure 24-21, tequila was diluted with water and extracted
Question:
In the analysis of odorants in tequila in Figure 24-21, tequila was diluted with water and extracted four times with dichloromethane (CH2Cl2, (CH2Cl2, b.p. 40°C)40°C). The 400 mL 400 mL of CH2Cl2CH2Cl2 was evaporated down to 1 mL, 1 mL, and 1 μL μL of the extract was injected on-column onto a poly(ethylene glycol) open tubular column (30 m longx0.53 mm(30 m long x 0.53 mm diameter, film thickness=1 μm thickness=1 μm) initially at 50°C 50°C and then ramped to 230°C. 230°C.
a. Why was the diluted tequila extracted four times with dichloromethane instead of once with a larger volume?
b. Why was on-column injection used?
c. Why was a poly(ethylene glycol) column chosen for this application?
d. What was the phase ratio of the column?
e. Why was a wide-bore 0.53-mm-diameter 0.53-mm-diameter column chosen for this application?
Figure 24-21
Step by Step Answer:

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy





