The densities (g/mL) of several substances are: acetic acid, 1.05; CCl 4 , 1.59; S, 2.07; Li,
Question:
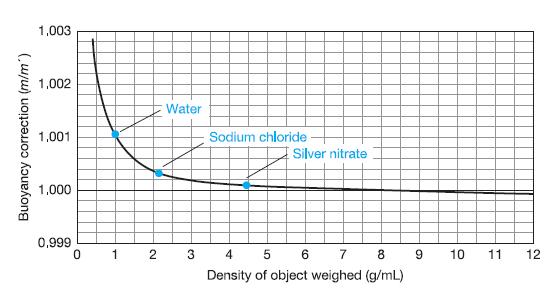
The densities (g/mL) of several substances are: acetic acid, 1.05; CCl4, 1.59; S, 2.07; Li, 0.53; Hg, 13.5; PbO2, 9.4; Pb, 11.4; Ir, 22.5. From Figure 2-9, predict which substances will have the smallest and largest buoyancy corrections.
Figure 2-9
Transcribed Image Text:
1,003 1,002 Water 1,001 Sodium chloride Silver nitrate 1,000 0,999 1 3 4 5 6 10 11 12 Density of object weighed (g/mL) Buoyancy correction (m/m') 2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (9 reviews)
Buoyancy correction factor m can be found as m 1 m is reading of mass balance da is density of air 0...View the full answer

Answered By

Abhinav Gupta
i am currently working on chegg as chemistry expert since 2018 . I have maintained a C.F. score of minimum 75% since the time I have started solving questions. I have also worked on topper tutors for 1 year for 2017 to 2018 with average 4 star ratings.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Related Video
Density helps us predict whether something will float. Density is super important to consider when building things like ships and submarines. The experiment in the video examines the water density by comparing a glass of water containing sugar with a simple glass of water
Students also viewed these Engineering questions
-
Acetic acid, CH3COOH, is contained in vinegar. Suppose acetic acid was formed from its elements, according to the following equation: 2C(graphite) + 2H2(g) + O2(g) CH3COOH(l) Find the enthalpy...
-
Ka for acetic acid is 1.7 10-5 at 25C. A buffer solution is made by mixing 52.1 mL of 0.122 M acetic acid with 46.1 mL of 0.182 M sodium acetate. Calculate the pH of this solution at 25C after the...
-
The distinctive odor of vinegar is due to acetic acid, CH3COOH, which reacts with sodium hydroxide in the following fashion: If 3.45 mL of vinegar needs 42.5 mL of 0.115 M NaOH to reach the...
-
LOCATE APPROPRIATE CPT CODES ICD-10-CM (CPT) FOR PROCEDURES BELOW- Outpatient procedures only 1. INCISION AND DRAINAGE OF A CYST --- 2. DEBRIDEMENT - 3. SIMPLE REPAIR OF A SUPERFICIAL WOUND- 4....
-
Toby Power, a fellow Illinois resident, is a new client. Prior to her telephone call this morning (late November), all you really knew about Toby was that for the past five years she has been earning...
-
Suppose that (n) of the points given to the closest-pair algorithm are covertical. Show how to determine the sets P L and P R and how to determine whether each point of Y is in P L or P R so that the...
-
Paula Phillips arrived back at her office at St. Paul Copy Machines around 4:00 on Tuesday afternoon. As she sat behind her desk looking dejected, her sales manager, Jeff Baker, showed up to ask how...
-
Stoner Excursions offers several services to customers. Susan Stoner realizes that some customers use more services than others, so the company has conducted a customer profitability analysis that...
-
table. 2. Design an 8-to-1 mux, 4-to-1 mux and 3-to-8 decoder to implement the following truth ABC F 000 1 0 0 1 0 0101 0110 1000 10 1 1101 11
-
Bruin Corporation has been authorized to issue 5,000 shares of 12 percent noncumulative, nonparticipating preferred stock with a par value of $100 per share and 200,000 shares of common stock with a...
-
Pentane (C 5 H 12 ) is a liquid with a density of 0.626 g/mL near 25C. Find the true mass of pentane when the mass in air is 14.82 g. Assume air density = 0.001 2 g/mL.
-
What do the symbols TD and TC mean on volumetric glassware?
-
If you invest the saving at the end of each month and earn 9% compounded monthly, how much (rounded to the nearest dollar) will you accumulate after: a. 20 years? b. 30 years? c. 40 years?
-
Use estimation to select the best response in Problems 7-24. Do not calculate. A reasonable APR to pay for a 3 -year installment loan is A. \(1 \%\) B. \(12 \%\) C. \(32 \%\)
-
Find the present value of the ordinary annuities in Problems 21-32. Amount of Deposit m 28. $400 Frequency n quarterly Rate r 11% Time t 20 yr
-
Use a calculator to evaluate an ordinary annuity formula \[A=m\left[\frac{\left(1+\frac{r}{n}ight)^{n t}-1}{\frac{r}{n}}ight]\] for \(m, r\), and \(t\) (respectively) given in Problems 7-22. Assume...
-
Explain summation notation.
-
a. Find the first three terms of the sequences whose nth terms are given. b. Classify the sequence as arithmetic (give d), geometric (give r), both, or neither. \(s_{n}=\frac{2}{3}\)
-
If you add a constraint to an optimization model, and the previously optimal solution satisfies the new constraint, will this solution still be optimal with the new constraint added? Why or why not?
-
Research corporate acquisitions using Web resources and then answer the following questions: Why do firms purchase other corporations? Do firms pay too much for the acquired corporation? Why do so...
-
Vernier scale. The figure below shows a scale found on instruments such as a micrometer caliper used for accurately measuring dimensions of objects. The lower scale slides along the upper scale and...
-
Controlling the appearance of a graph. Figure 3-3 requires gridlines to read buret corrections. In this exercise, you will format a graph so that it looks like Figure 3-3. Follow the procedure in...
-
Why do we use quotation marks around the word true in the statement that accuracy refers to how close a measured value is to the true value?
-
4. A partnership owns an aging 4-unit retail center in a local campus property. Cash flow projections for the next 10 years are: $50,000 for years 1 and 2; $60,000 for years 3 and 4; $70,000 for...
-
given A = 2 -2 3 ' 3 4 2 , 4 2 5 2 2 B= 3 -3 2 2 1 2 1 4 2 2 3 F= 4 2 3 - 2 -2 4 2 3 2 3 4 2 4 I Find the resulting matricas based on the arth metic operation. attach solution on your the comment...
-
The 40 members of a recreation class were asked to name their favorite sports. The table shows the numbers who responded in various ways. Use information given in the table to answer the following...

Study smarter with the SolutionInn App


