Question: We have a 37.0 ( 0.5) wt% HCl solution with a density of 1.18 ( 0.01) g/mL. To deliver 0.050 0 mol of HCl requires
We have a 37.0 (± 0.5) wt% HCl solution with a density of 1.18 (± 0.01) g/mL. To deliver 0.050 0 mol of HCl requires 4.18 mL of solution. If the uncertainty that can be tolerated in 0.050 0 mol is ±2%, how big can the absolute uncertainty in 4.18 mL be?
Caution: In this problem, you have to work backward. You would normally compute the uncertainty in mol HCl from the uncertainty in volume:

But, in this case, we know the uncertainty in mol HCl (2%) and we need to find what uncertainty in mL solution leads to that 2% uncertainty. The arithmetic has the form a = b × c × d, for which
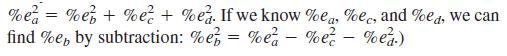
mL solution X g solution g HCI mol HCl mL solution g solution g HCI mol HCI
Step by Step Solution
3.48 Rating (164 Votes )
There are 3 Steps involved in it
Percentage uncertainity tolerated in mol HCl e a 2 Un... View full answer

Get step-by-step solutions from verified subject matter experts


