Question: Which is a stronger acid, (a) or (b)? (a) (b) Cl,HCCOH , Dichloroacetic acid Chloroacetic acid K = 8 x 10-2 K = 1.36 x
Which is a stronger acid, (a) or (b)?
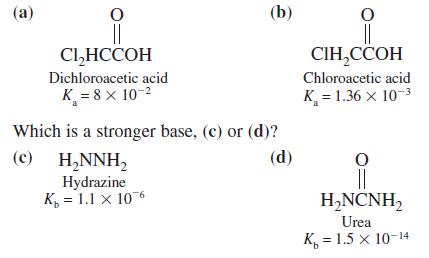
(a) (b) Cl,HCCOH , Dichloroacetic acid Chloroacetic acid K = 8 x 10-2 K = 1.36 x 10-3 Which is a stronger base, (c) or (d)? (c) (d) H,NNH, Hydrazine K, = 1.1 x 106 H,NCNH, Urea K, = 1.5 x 10-14
Step by Step Solution
3.40 Rating (163 Votes )
There are 3 Steps involved in it
Since Ka is acid dissociation constant so hi... View full answer

Get step-by-step solutions from verified subject matter experts


