Given the following information: Energy of sublimation of Na(s) = 97 kJ/mol Bond energy of HBr =
Fantastic news! We've Found the answer you've been seeking!
Question:
Given the following information:
Energy of sublimation of Na(s) = 97 kJ/mol
Bond energy of HBr = 363 kJ/mol
Ionization energy of Na (g) = 496 kJ/mol
Electron affinity of Br (g) = ?324 kJ/mol
Lattice energy of NaBr (s) = ?781 kJ/mol
Bond energy of H, = 432 kJ/mol
Calculate the net change in energy for the following reaction:
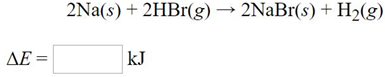
Related Book For 

Fraud examination
ISBN: 978-0538470841
4th edition
Authors: Steve Albrecht, Chad Albrecht, Conan Albrecht, Mark zimbelma
Posted Date:




