Research is being carried out on cellulose as a source of chemicals for the production of fibers,
Fantastic news! We've Found the answer you've been seeking!
Question:
Research is being carried out on cellulose as a source of chemicals for the production of fibers, coatings, and plastics. Cellulose consists of long chains of glucose molecules (C6H12O6), so for the purposes of modeling the reaction we can consider the conversion of glucose to formaldehyde (H2CO). Calculate the heat of reaction for the conversion of 1 mole of glucose into formaldehyde, given the following thermochemical data:
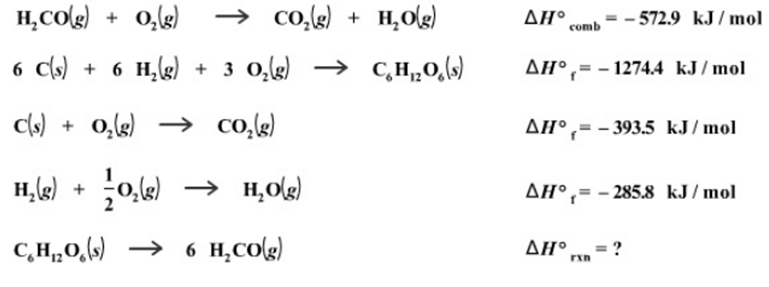
Related Book For 

Manufacturing Processes for Engineering Materials
ISBN: 978-0132272711
5th edition
Authors: Serope Kalpakjian, Steven Schmid
Posted Date:




