There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several
Fantastic news! We've Found the answer you've been seeking!
Question:
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. In the first step, calcium carbide and water react to form acetylene and calcium hydroxide:
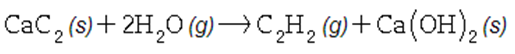
In the second step, acetylene, carbon dioxide and water react to form acrylic acid:
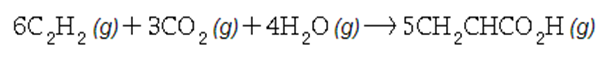
Write the net chemical equation for the production of acrylic acid from calcium carbide, water and carbon dioxide. Be sure your equation is balanced.
Related Book For 

Auditing Cases An Interactive Learning Approach
ISBN: 978-0133852103
6th edition
Authors: Mark S. Beasley, Frank A. Buckless, Steven M. Glover, Douglas F. Prawitt
Posted Date:




