Question: When 1.23 g of menthol is dissolved in 5.00 mL of ether and placed in a sample cell 10.0 cm in length, the observed
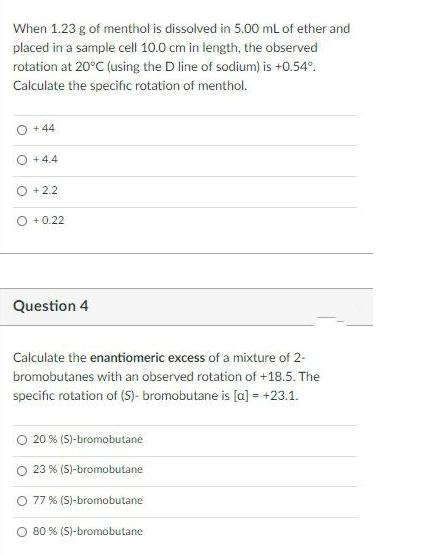
When 1.23 g of menthol is dissolved in 5.00 mL of ether and placed in a sample cell 10.0 cm in length, the observed rotation at 20C (using the D line of sodium) is +0.54. Calculate the specific rotation of menthol. O + 44 +4.4 O + 2.2 O + 0.22 Question 4 Calculate the enantiomeric excess of a mixture of 2- bromobutanes with an observed rotation of +18.5. The specific rotation of (S)- bromobutane is [a] = +23.1. 20 % (S)-bromobutane O 23 % (S)-bromobutane O 77% (S)-bromobutane 80 % (S)-bromobutane
Step by Step Solution
3.46 Rating (159 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


