Question: Consider the following system at equilibrium where AH 92.7 kJ, and K. =1.80x10 at 298 K NH,HS()NH3(g) + H,S(g) When some moles of NH
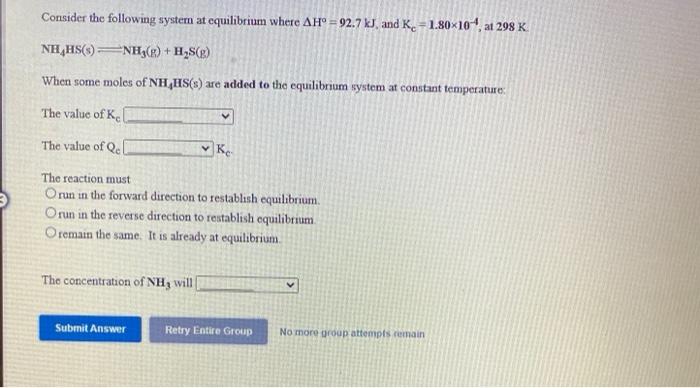
Consider the following system at equilibrium where AH 92.7 kJ, and K. =1.80x10 at 298 K NH,HS()NH3(g) + H,S(g) When some moles of NH HS(s) are added to the equilibrium system at constant temperature: The value of K. The value of Qe Ke The reaction must Orun in the forward direction to restablsh equilibrium. Orun in the reverse direction to restablish equilibrium O remain the same. It is already at equilibrium. The concentration of NH3 wil Submit Answer Retry Entire Group No more group attempts remain
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
The equilibrium reaction is NH4HSs NH3g H2Sg at equilibriu... View full answer

Get step-by-step solutions from verified subject matter experts


