Question: What is the ground-state electron configuration of Fe3+? Answer with whole numbers only. If the subshell has no electrons, enter a zero. 4p 4s
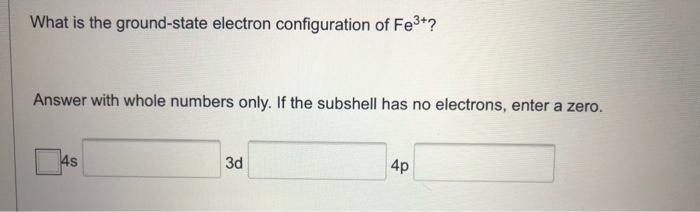
What is the ground-state electron configuration of Fe3+? Answer with whole numbers only. If the subshell has no electrons, enter a zero. 4p 4s 3d
Step by Step Solution
3.42 Rating (142 Votes )
There are 3 Steps involved in it
The electronic configuration of Fe is 1s2 2s2 2p6 3s2 3p6 ... View full answer

Get step-by-step solutions from verified subject matter experts


