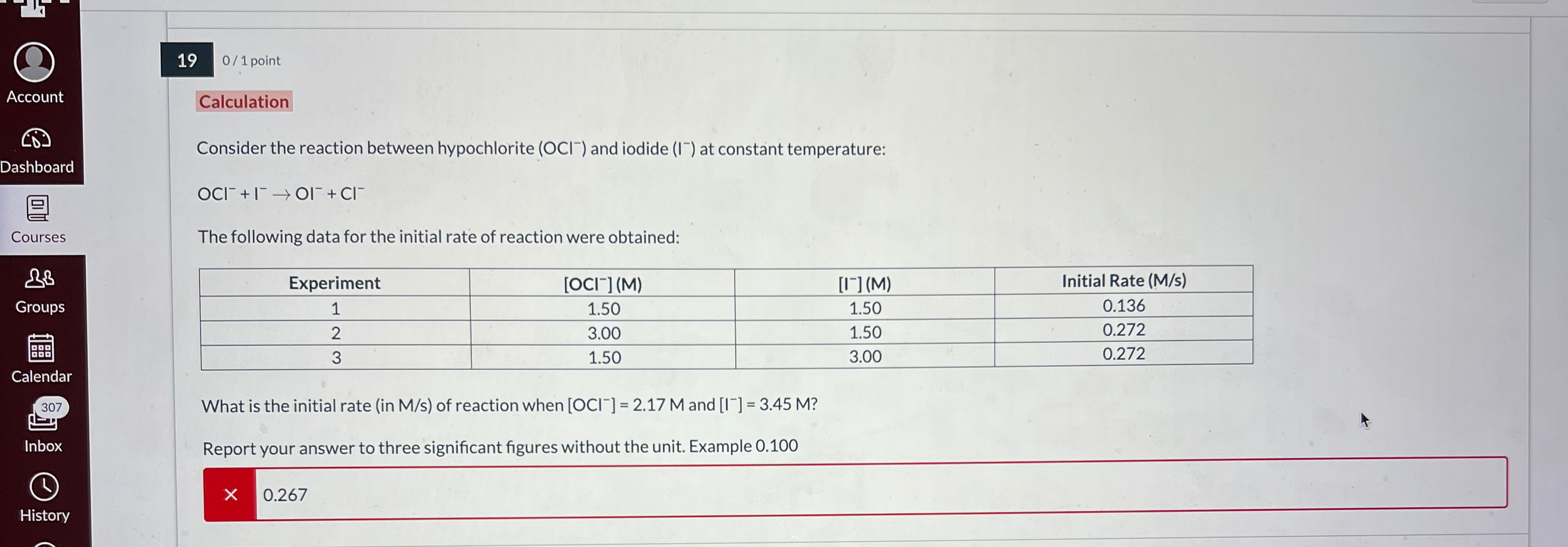
Question: 0 1 point Calculation Consider the reaction between hypochlorite ( O C l - ) and iodide ( I - ) at constant temperature: O
point
Calculation
Consider the reaction between hypochlorite and iodide at constant temperature:
Courses
The following data for the initial rate of reaction were obtained:
tableExperimentInitial Rate Ms
What is the initial rate in Ms of reaction when and
Report your answer to three significant figures without the unit. Example
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


