Question: + +0 2. Suppose an electrochemical cell was constructed using two redox couples, methylviologen2+/ (MV2+/+, E = -0.64 V vs NHE) and ferrocene/+ (Fc+/0, E
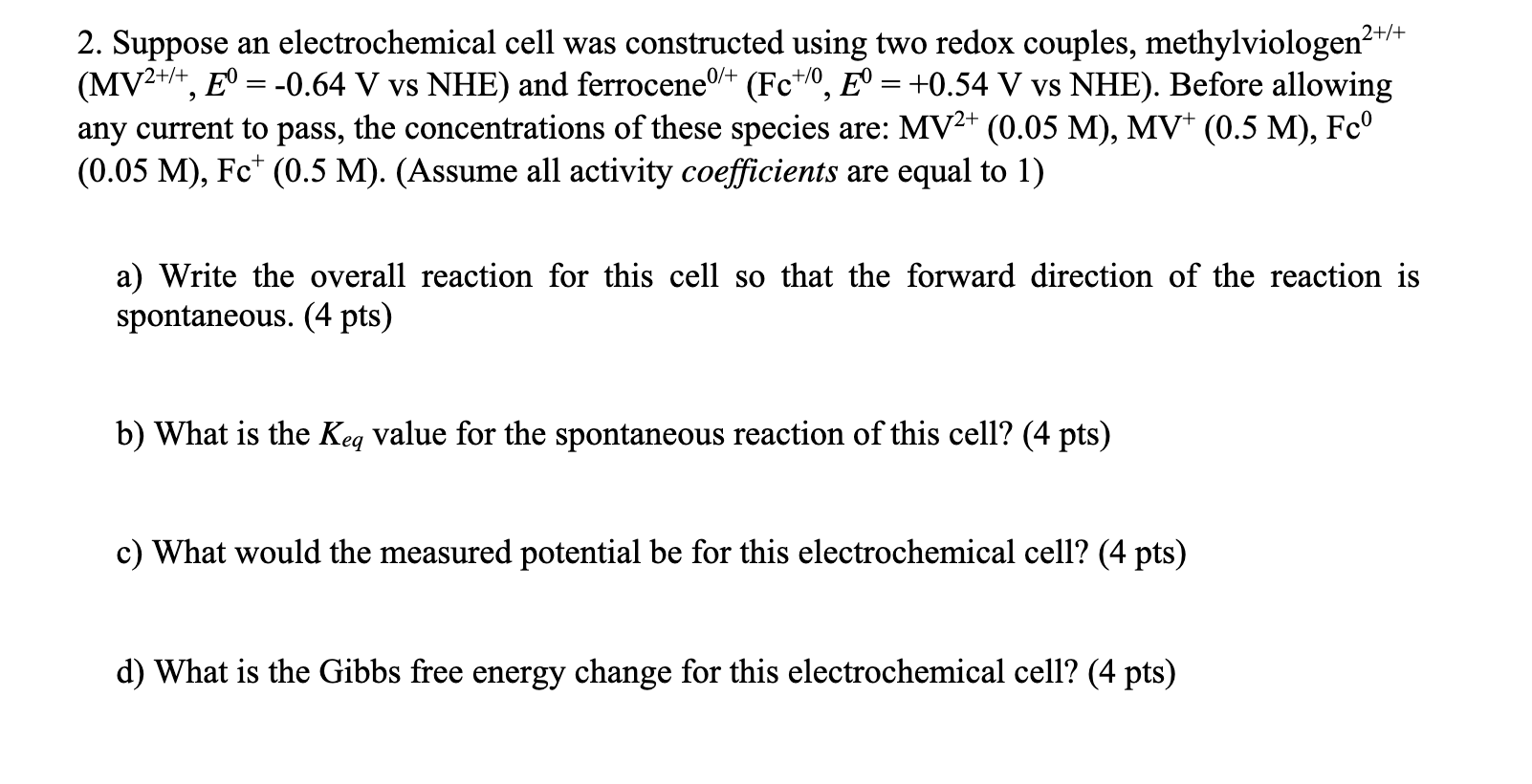
+ +0 2. Suppose an electrochemical cell was constructed using two redox couples, methylviologen2+/ (MV2+/+, E = -0.64 V vs NHE) and ferrocene/+ (Fc+/0, E = +0.54 V vs NHE). Before allowing any current to pass, the concentrations of these species are: MV2+ (0.05 M), MV+ (0.5 M), Fc (0.05 M), Fc (0.5 M). (Assume all activity coefficients are equal to 1) a) Write the overall reaction for this cell so that the forward direction of the reaction is spontaneous. (4 pts) b) What is the Keq value for the spontaneous reaction of this cell? (4 pts) c) What would the measured potential be for this electrochemical cell? (4 pts) d) What is the Gibbs free energy change for this electrochemical cell? (4 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


