Question: 0 eV n = oo -0.54 eV n= 5 -0.85 eV n = 4 -1.51 eV n = 3 -3.40 eV n = 2 -13.6
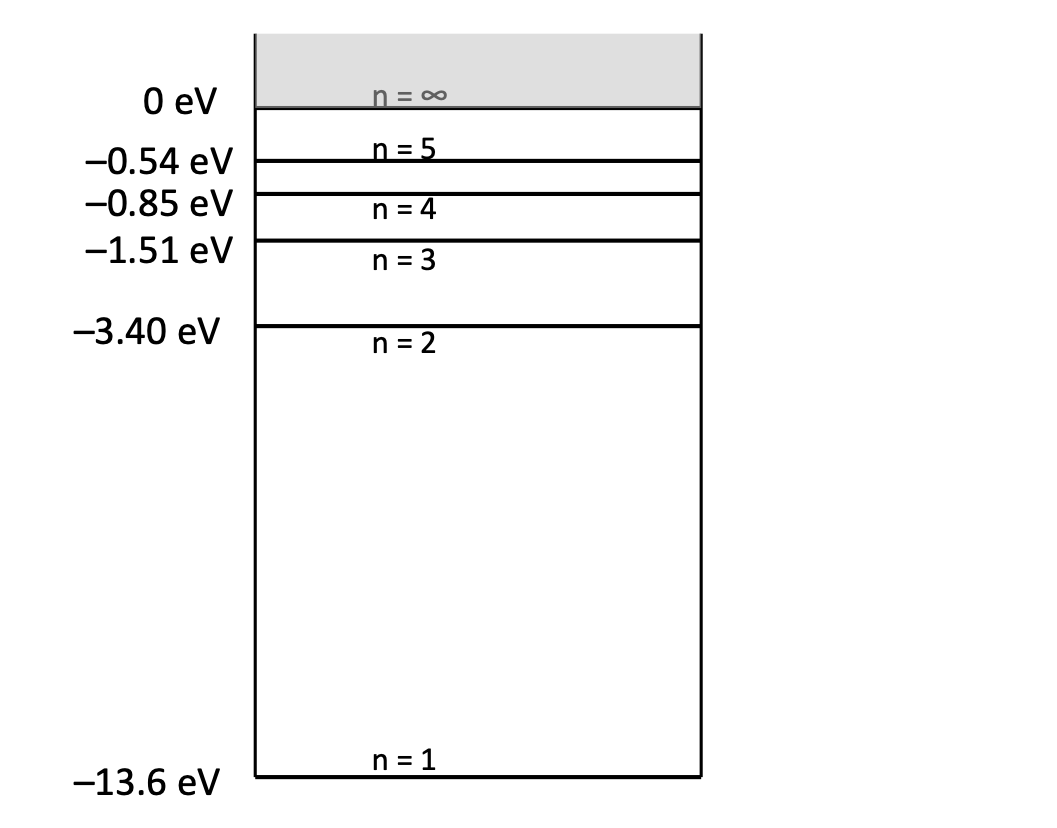
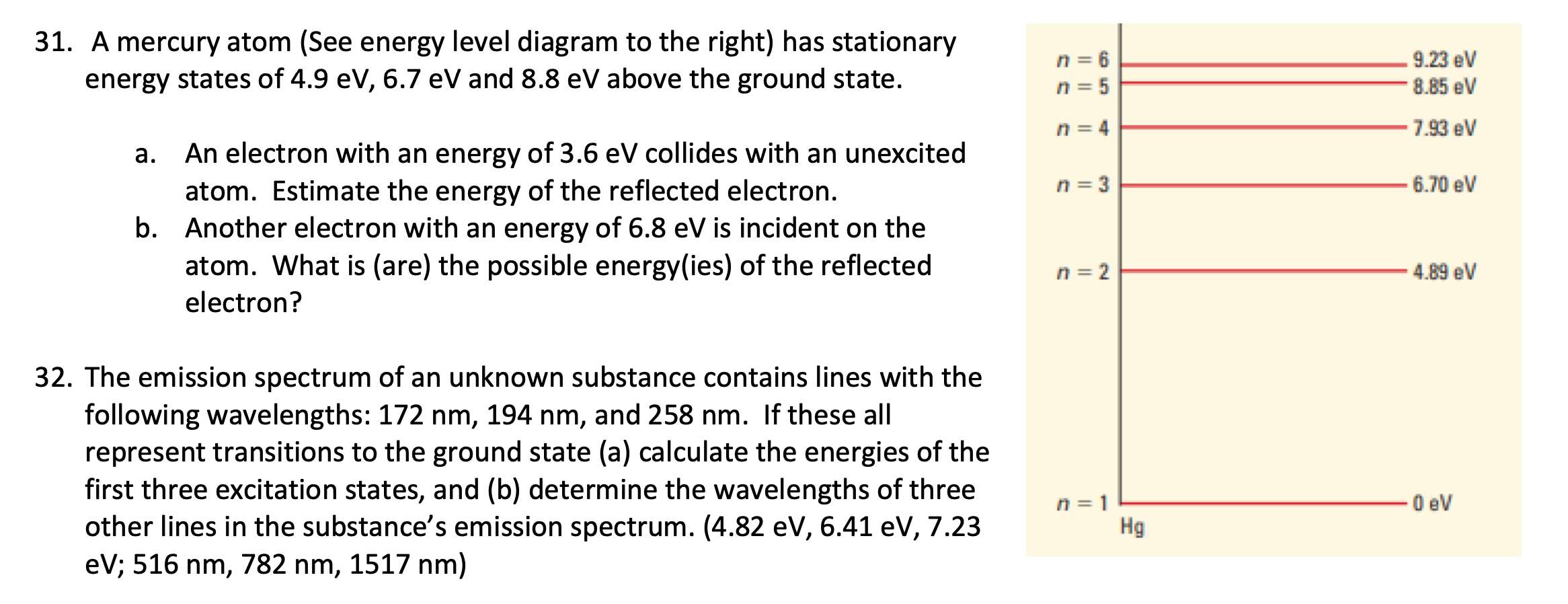
0 eV n = oo -0.54 eV n= 5 -0.85 eV n = 4 -1.51 eV n = 3 -3.40 eV n = 2 -13.6 eV n = 131. A mercury atom (See energy level diagram to the right) has stationary n = 6 9.23 eV energy states of 4.9 eV, 6.7 eV and 8.8 ev above the ground state. n = 5 8.85 eV 1 = 4 7.93 eV a. An electron with an energy of 3.6 ev collides with an unexcited atom. Estimate the energy of the reflected electron. n = 3 6.70 ev b. Another electron with an energy of 6.8 ev is incident on the atom. What is (are) the possible energy(ies) of the reflected n = 2 4.89 ev electron? 32. The emission spectrum of an unknown substance contains lines with the following wavelengths: 172 nm, 194 nm, and 258 nm. If these all represent transitions to the ground state (a) calculate the energies of the first three excitation states, and (b) determine the wavelengths of three n = 1 0 ev other lines in the substance's emission spectrum. (4.82 eV, 6.41 ev, 7.23 Hg eV; 516 nm, 782 nm, 1517 nm)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


