Question: 0.008 4) Using an appropriate mixing rule, Calculate and plot the z factor for the gas composition given below at several pressures and 160 F.
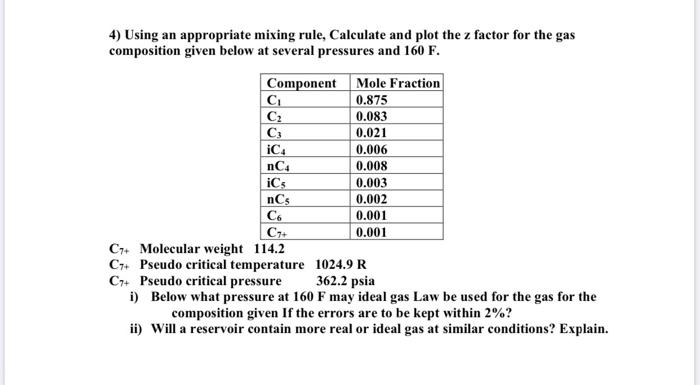
0.008 4) Using an appropriate mixing rule, Calculate and plot the z factor for the gas composition given below at several pressures and 160 F. Component Mole Fraction C 0.875 0.083 C 0.021 iC4 0.006 nC4 iCs 0.003 nCs 0.002 C6 0.001 C++ 0.001 C7+ Molecular weight 114.2 C7+ Pseudo critical temperature 1024.9 R C7+ Pseudo critical pressure 362.2 psia i) Below what pressure at 160 F may ideal gas Law be used for the gas for the composition given If the errors are to be kept within 2%? ii) Will a reservoir contain more real or ideal gas at similar conditions? Explain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


