Question: 02) An absorption column, operating at 4.5 bar and 30C, is applied to separate SO2 (A) from air (B) using water as an absorbent. At
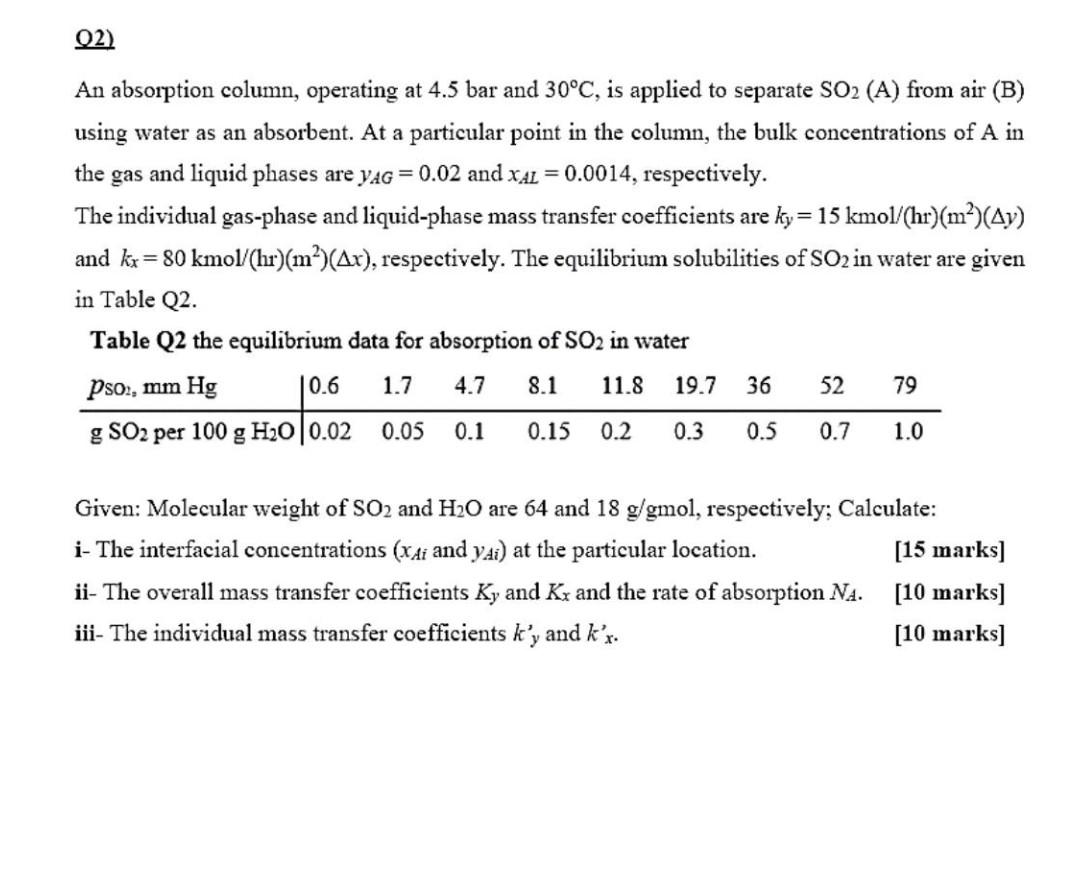
02) An absorption column, operating at 4.5 bar and 30C, is applied to separate SO2 (A) from air (B) using water as an absorbent. At a particular point in the column, the bulk concentrations of A in the gas and liquid phases are yAG = 0.02 and XAL = 0.0014, respectively. The individual gas-phase and liquid-phase mass transfer coefficients are ky= 15 kmol/(hr)(m)(Ay) and kx = 80 kmol/(h)(m)(Ar), respectively. The equilibrium solubilities of SO2 in water are given in Table Q2. Table Q2 the equilibrium data for absorption of SO2 in water Pso2, mm Hg 0.6 8.1 11.8 19.7 36 52 79 g SO2 per 100 g H20|0.02 0.05 0.1 0.15 0.2 0.3 0.5 0.7 1.0 1.7 4.7 Given: Molecular weight of SO2 and H2O are 64 and 18 g/gmol, respectively; Calculate: i- The interfacial concentrations (Xti and ydi) at the particular location. [15 marks] ii- The overall mass transfer coefficients Ky and Ky and the rate of absorption NA. [10 marks] iii- The individual mass transfer coefficients ky and k'r. [10 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


