Question: 0.2 From the information in the table below, calculate the standard Gibbs energy and the equilibrium constant at (a) 25C and (b) 50C for the
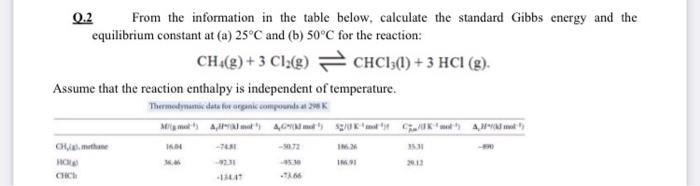
0.2 From the information in the table below, calculate the standard Gibbs energy and the equilibrium constant at (a) 25C and (b) 50C for the reaction: CH() + 3 C128) CHC13(1) + 3 HCl (g). Assume that the reaction enthalpy is independent of temperature. Thermodynamic data for organic compounds 29 Memet A40%" at SUKA CHU. I - -0.73 IM IM 2013 Chin 11 966
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


