Question: 0.9. Consider equilibrium between two phases in a single-component system. Show that the change in chemical potential with pressure along the phase boundary is given
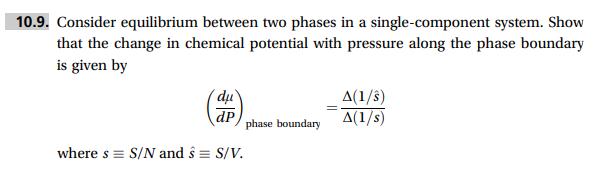
0.9. Consider equilibrium between two phases in a single-component system. Show that the change in chemical potential with pressure along the phase boundary is given by (dPd)phaseboundary=(1/s)(1/s^) where sS/N and s^S/V
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


