Question: 1 0 . 3 1 . Repeat Example 1 0 . 1 4 using water as the absorbing liquid. ( a ) Show that the
Repeat Example using water as the absorbing liquid.
a Show that the solubility of toluene in water at listed in Table corresponds to a Henry's law constant of ~~atm. Assume that this value at is applicable also at only a fair assumption!
b Then compute and from it
c Comment on the feasibility o
f using water
this is the solution of example
in this example
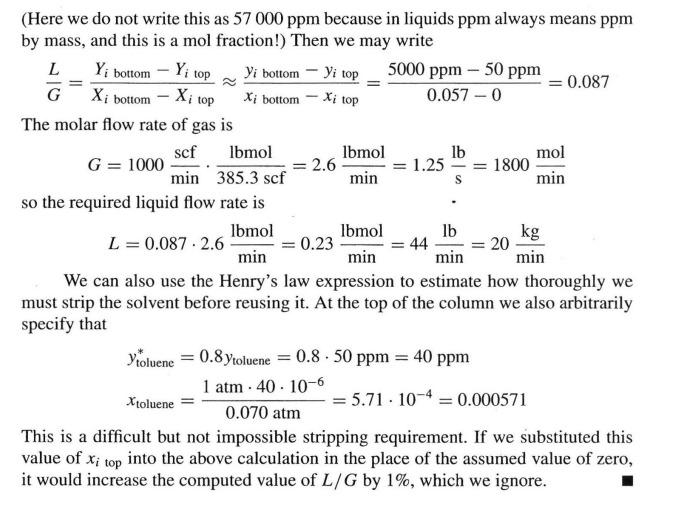
Here we do not write this as because in liquids ppm always means ppm by mass, and this is a mol fraction! Then we may write
~~
The molar flow rate of gas is
so the required liquid flow rate is
We can also use the Henry's law expression to estimate how thoroughly we must strip the solvent before reusing it At the top of the column we also arbitrarily specify that
This is a difficult but not impossible stripping requirement. If we substituted this value of top into the above calculation in the place of the assumed value of zero, it would increase the computed value of by which we ignore.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


