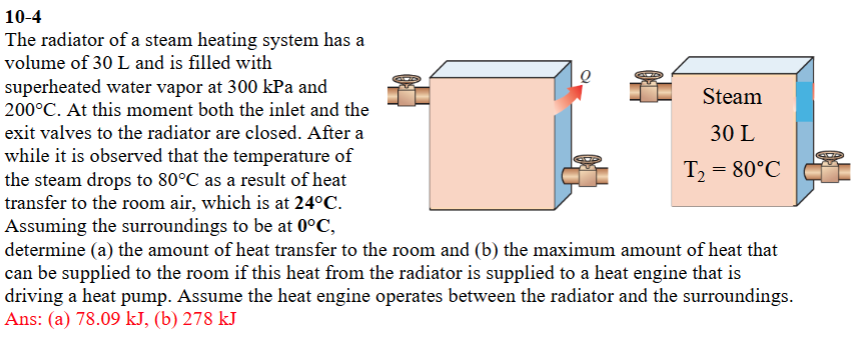
Question: ( 1 0 - 4 ) The radiator of a steam heating system has a volume of 3 0 L and is filled
The radiator of a steam heating system has a volume of L and is filled with superheated water vapor at kPa and circmathrmC At this moment both the inlet and the exit valves to the radiator are closed. After a while it is observed that the temperature of the steam drops to circmathrmC as a result of heat transfer to the room air, which is at circmathrmC Assuming the surroundings to be at circmathrmC determine a the amount of heat transfer to the room and b the maximum amount of heat that can be supplied to the room if this heat from the radiator is supplied to a heat engine that is driving a heat pump. Assume the heat engine operates between the radiator and the surroundings. Ans: a kJ b kJ
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


