Question: 1 0 Question ( 1 point ) See page 9 1 7 The oxidation of N H 4 + to N O 3 - in
Question point
See page
The oxidation of to in acid solution is described by the following equation:
The overall reaction for the oxidation has an
for the halfreaction
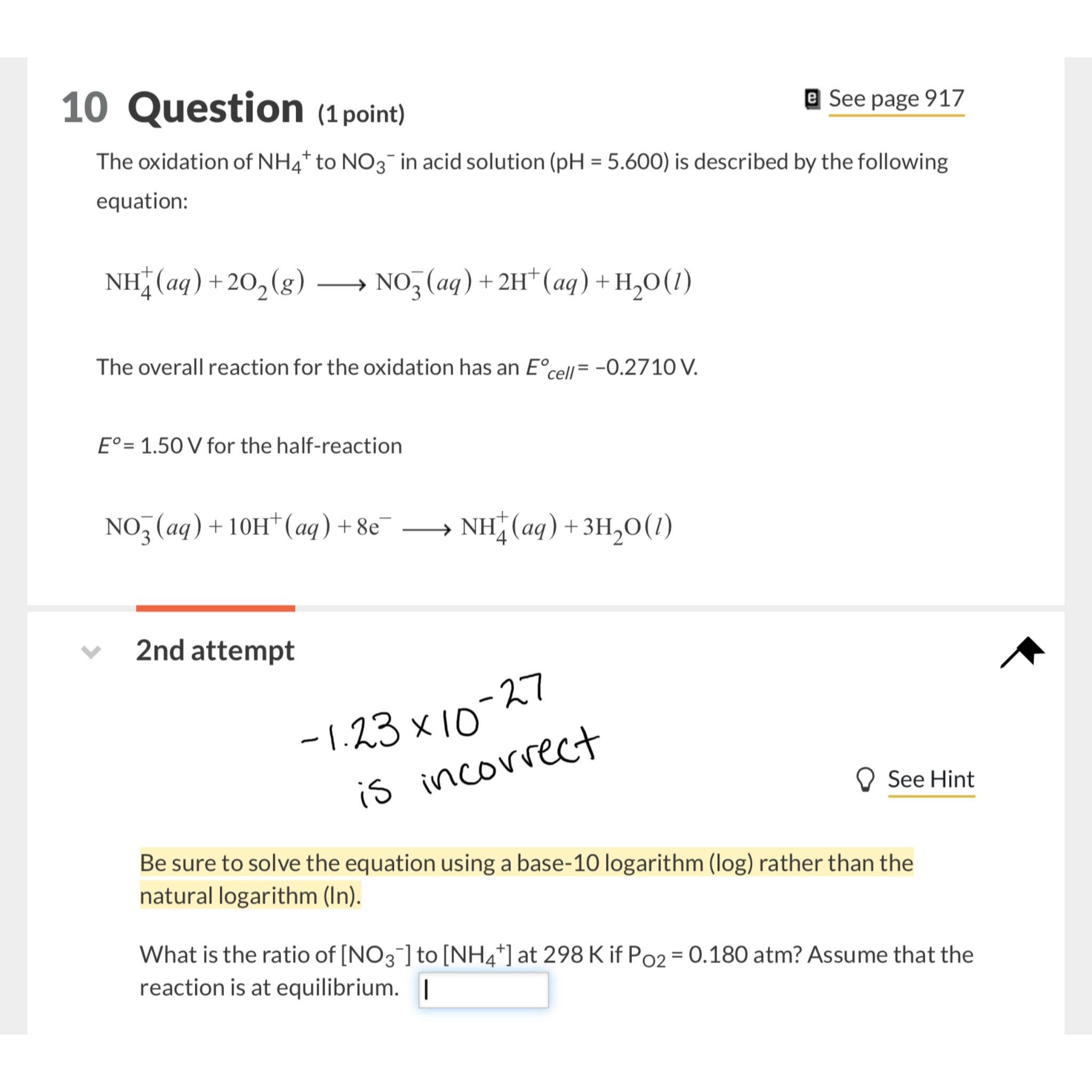
nd attempt
See Hint
Be sure to solve the equation using a base logarithm rather than the natural logarithm In
What is the ratio of to at if atm? Assume that the reaction is at equilibrium.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


