Question: 1 1 1 ( ) 1 , 1 8 , 1 0 0 ( 1 0 % ) Explain the following items: ( b )
Explain the following items:
b JouleThomson coefficient
a Law of corresponding states
b Jour
e Ideal solutions
c Chemical potential
d The second law of thermodynamic
e Ideal solutions
Three moles of an ideal gas expand isothermally and reversibly from to at Calculate the w q and for this system.
a A reaction BharrZ has an equilibrium constant of at and a value of Calculate the entropy change for the reaction at b If the and values are temperature independent, at what temperature is the equilibrium constant equal to
The vapor pressure for pure water at is and at is Use these data to calculate the change in enthalpy per mol for the vaporization process.
Calculate the mole fraction, activity, and activity coefficients for water when gNaCl are dissolved in water at The molecular weight of NaCl is The vapor pressure is kPa.
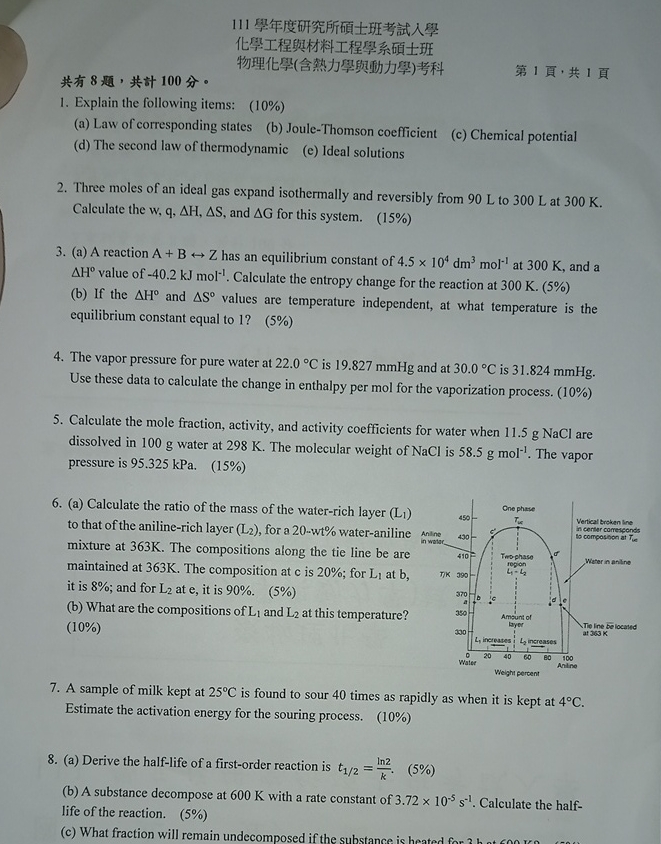
a Calculate the ratio of the mass of the waterrich layer to that of the anilinerich layer for a w wt wateraniline mixture at The compositions along the tie line be are maintained at The composition at is ; for at it is ; and for at it is
b What are the compositions of and at this temperature?
A sample of milk kept at is found to sour times as rapidly as when it is kept at Estimate the activation energy for the souring process.
a Derive the halflife of a firstorder reaction is
b A substance decompose at with a rate constant of Calculate the halflife of the reaction.
c What fraction will remain undecomposed if the
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


