Question: 1 1 : 1 5 9 2 Discussion 3 _ CED 3 6 0 1 copy What to consider Overall material balance Entering: Ammonia +
:
Discussion CED copy
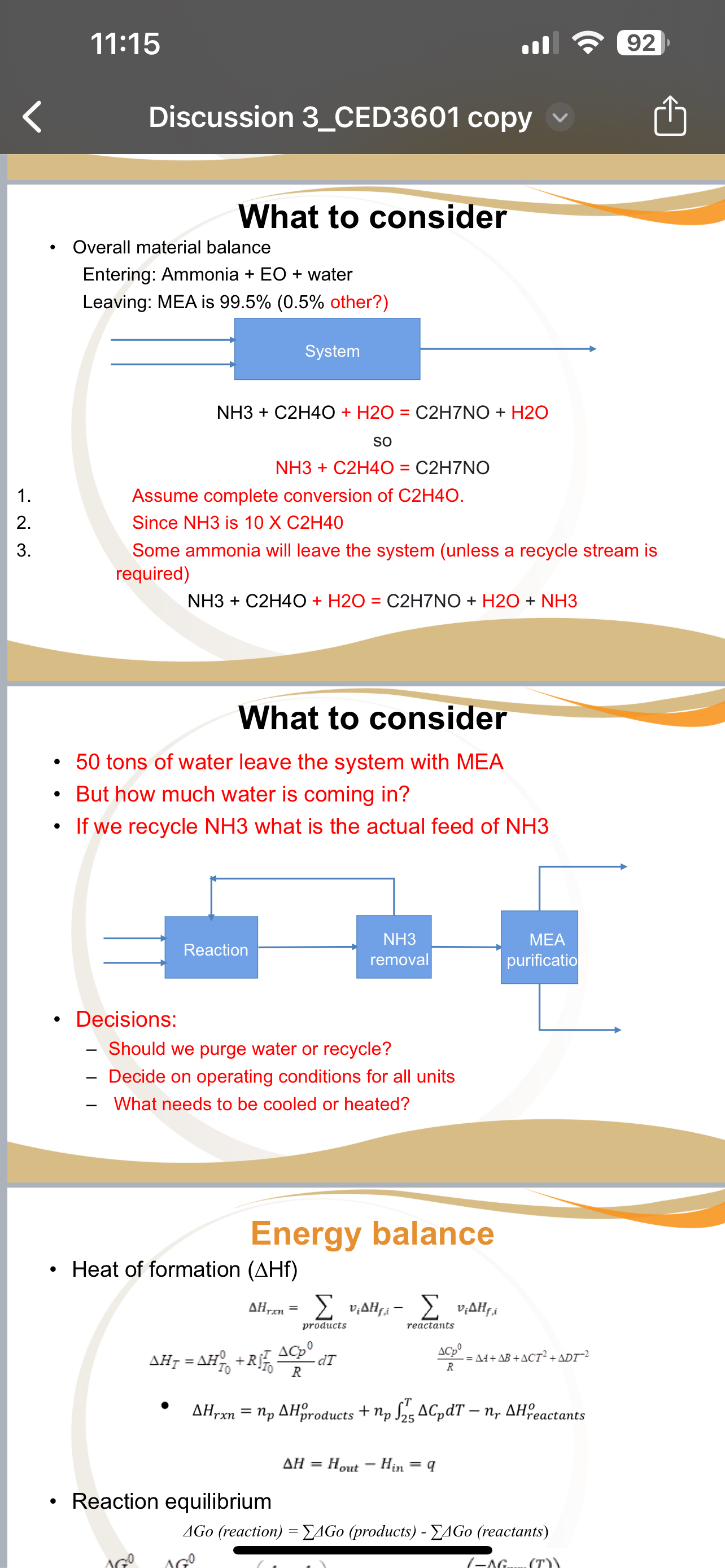
What to consider
Overall material balance
Entering: Ammonia water
Leaving: MEA is other?
System
Assume complete conversion of
Since NH is
Some ammonia will leave the system unless a recycle stream is required
What to consider
tons of water leave the system with MEA
But how much water is coming in
If we recycle what is the actual feed of
Decisions:
Should we purge water or recycle?
Decide on operating conditions for all units
What needs to be cooled or heated?
Energy balance
Heat of formation
Reaction equilibrium
reaction
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


