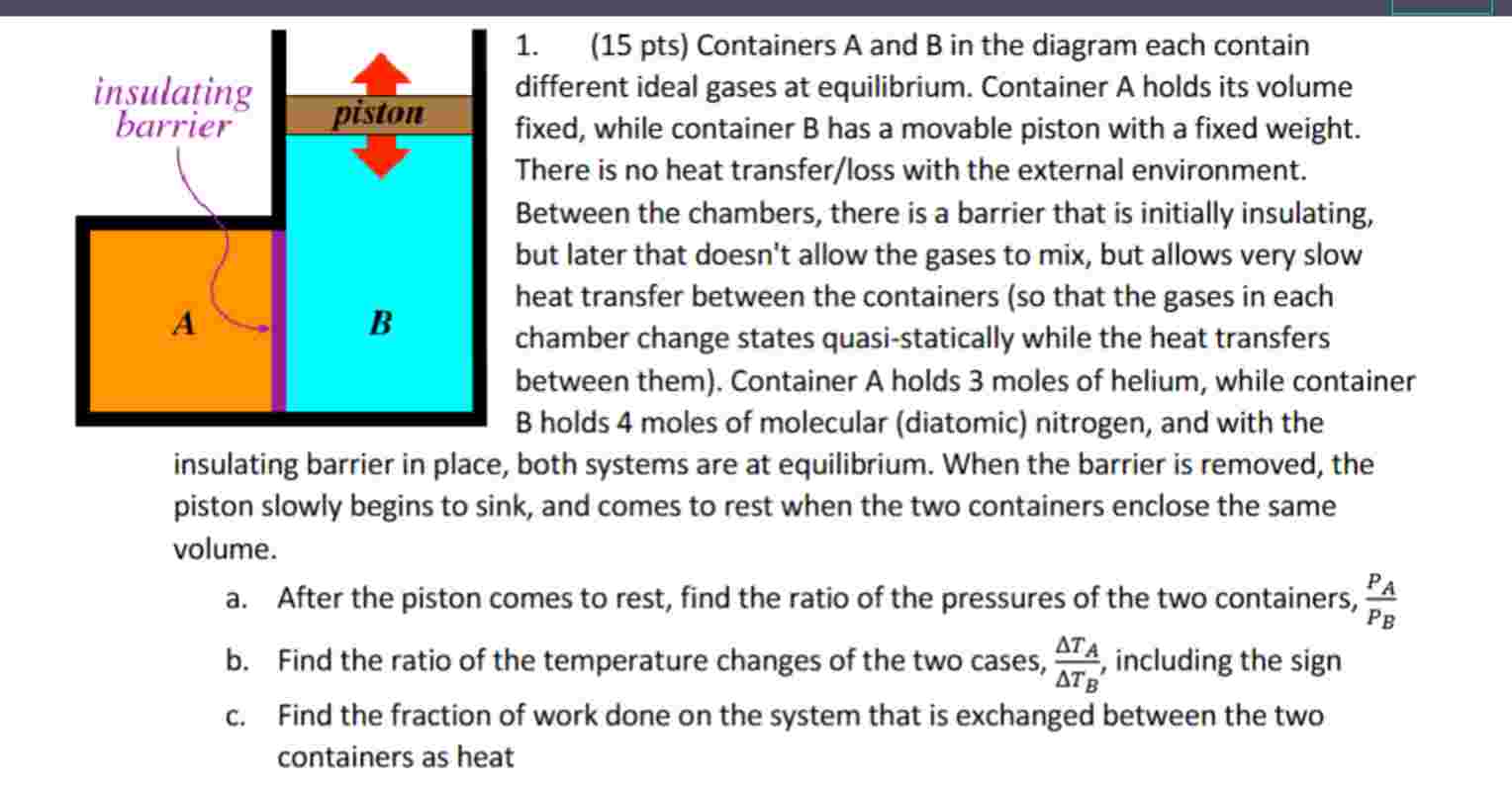
Question: 1 . ( 1 5 pts ) Containers ( A ) and ( B ) in the diagram each contain different
pts Containers A and B in the diagram each contain different ideal gases at equilibrium. Container A holds its volume fixed, while container B has a movable piston with a fixed weight. There is no heat transferloss with the external environment. Between the chambers, there is a barrier that is initially insulating, but later that doesn't allow the gases to mix, but allows very slow heat transfer between the containers so that the gases in each chamber change states quasistatically while the heat transfers between them Container A holds moles of helium, while container B holds moles of molecular diatomic nitrogen, and with the insulating barrier in place, both systems are at equilibrium. When the barrier is removed, the piston slowly begins to sink and comes to rest when the two containers enclose the same volume.
a After the piston comes to rest, find the ratio of the pressures of the two containers, fracPAPB
b Find the ratio of the temperature changes of the two cases, fracDelta TADelta TB including the sign
c Find the fraction of work done on the system that is exchanged between the two containers as heat
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


