Question: -1 -1 Paragraph for Question 5 & 6 X and Y are two volatile liquids with molar weights of 10g mol and 40g mol
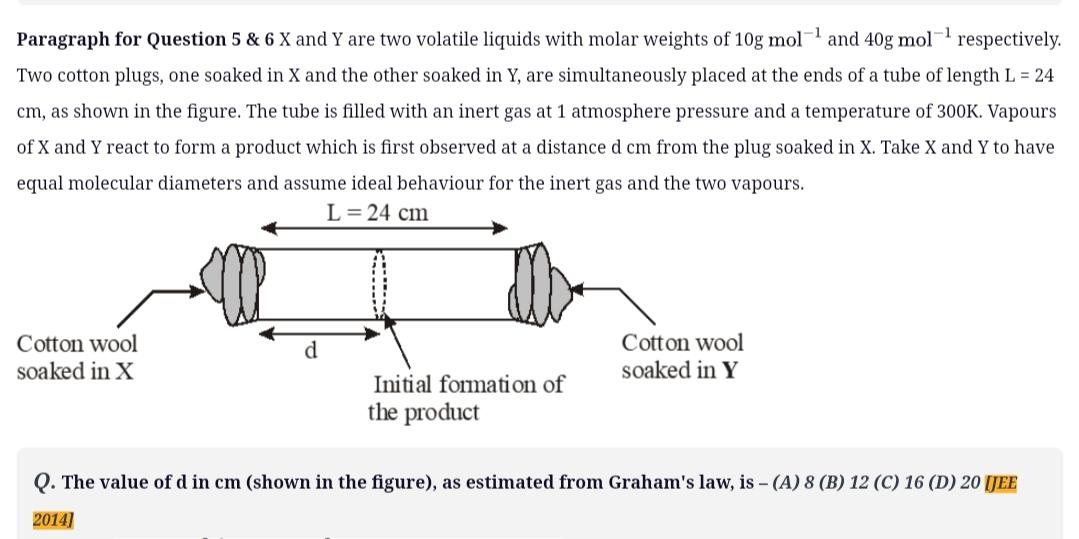
-1 -1 Paragraph for Question 5 & 6 X and Y are two volatile liquids with molar weights of 10g mol and 40g mol respectively. Two cotton plugs, one soaked in X and the other soaked in Y, are simultaneously placed at the ends of a tube of length L = 24 cm, as shown in the figure. The tube is filled with an inert gas at 1 atmosphere pressure and a temperature of 300K. Vapours of X and Y react to form a product which is first observed at a distance d cm from the plug soaked in X. Take X and Y to have equal molecular diameters and assume ideal behaviour for the inert gas and the two vapours. L = 24 cm Cotton wool soaked in X d Initial formation of the product Cotton wool soaked in Y Q. The value of d in cm (shown in the figure), as estimated from Graham's law, is - (A) 8 (B) 12 (C) 16 (D) 20 [JEE 2014]
Step by Step Solution
3.35 Rating (161 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


