Question: 1. (18 pts) Write one Lewis (electron dot) structure for the following molecules. a) AsF4 Explanation_ .. 4-2-4 =N-CI: F AS- b) ASF4 5+4
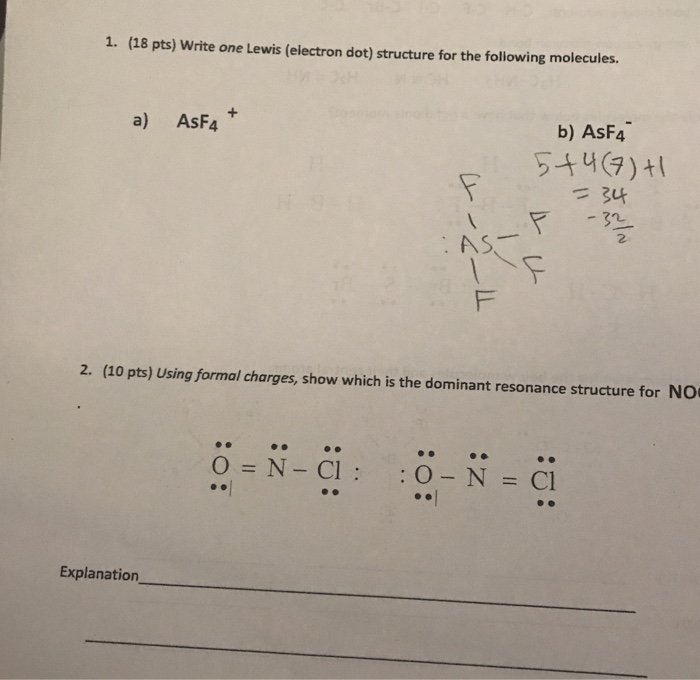
1. (18 pts) Write one Lewis (electron dot) structure for the following molecules. a) AsF4 Explanation_ .. 4-2-4 =N-CI: F AS- b) ASF4 5+4 (7)+1 = 34 2. (10 pts) Using formal charges, show which is the dominant resonance structure for NO 11 :2: -3 : 0- N = Cl =
Step by Step Solution
3.43 Rating (162 Votes )
There are 3 Steps involved in it
1 ASF 2 AS FO F A F 1 A5 4 electrony 7 electrons F valance electory As 6 electing F 7 elect... View full answer

Get step-by-step solutions from verified subject matter experts


