Question: 1. 2. 3. 1A BA H 2A 3A 4A SA 6A A He Be BCN O F Ne Na Mg x 40 sec 78 0
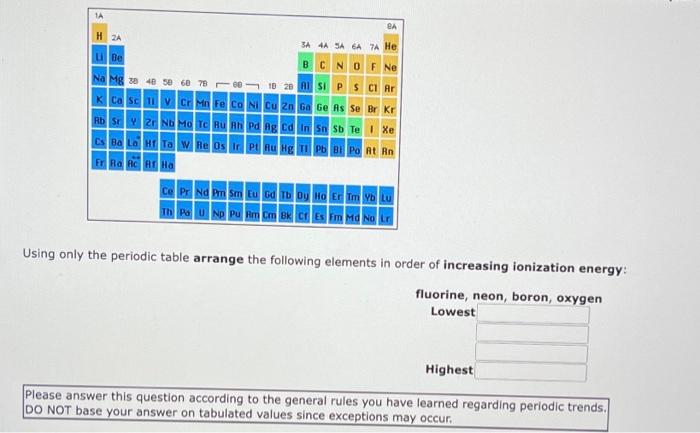
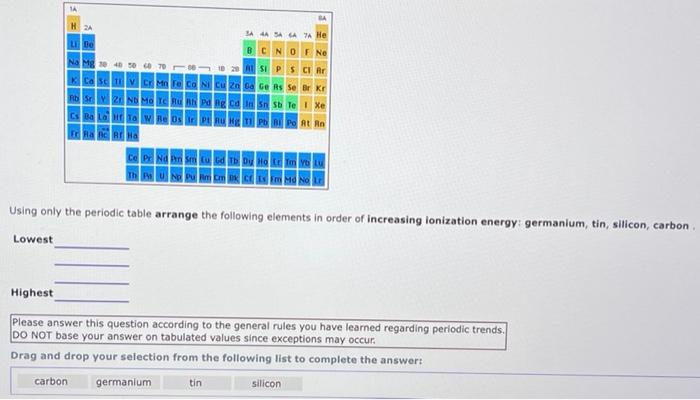
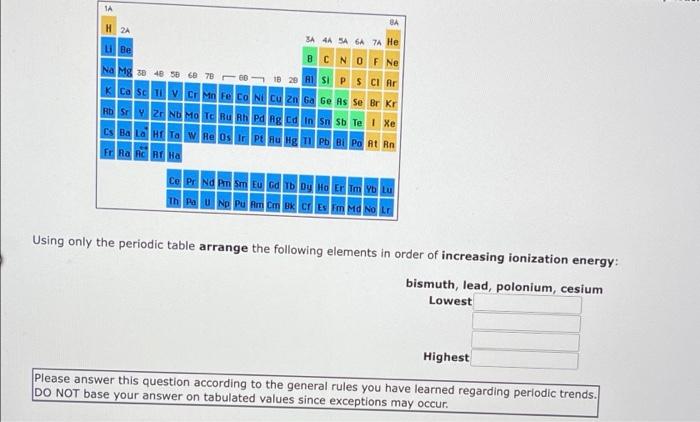
1A BA H 2A 3A 4A SA 6A A He Be BCN O F Ne Na Mg x 40 sec 78 0 23 ASI P S Cl Ar K Ca Sc in Fe Co Ni Cu Zn Ga Ge As Se Br Kr | HD | 2 Nh Ma Tc Bu Ph Pa lg Edin 5 Te | Xe Cs Ba LG Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn ERA AC AT HA Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm YOLU Using only the periodic table arrange the following elements in order of increasing ionization energy: fluorine, neon, boron, oxygen Lowest Highest Please answer this question according to the general rules you have learned regarding periodic trends. DO NOT base your answer on tabulated values since exceptions may occur. ube Na Mg ca MM CONCM 2 ca Bad & O F Ne SIPSCI Po At Rn CE PENA Thu Using only the periodic table arrange the following elements in order of increasing ionization energy: germanium, tin, silicon, carbon Lowest Highest Please answer this question according to the general rules you have learned regarding periodic trends. DO NOT base your answer on tabulated values since exceptions may occur. Drag and drop your selection from the following list to complete the answer carbon germanium silicon tin 1A BA H 2A SA SA 6A 7 He u Be B C N O F Ne Na Mg 30 40 50 60 78 66 to 29 ASI P S Cl Ar K Ca Sc V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr H | 2 N Mo T hu Bh v ng ta n n sb Te | Xe Cs Ba LG HET W Re Os PU Bug 11 Pb Bi Po At Rn FRA AC A Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am C B C Em Md No LG Using only the periodic table arrange the following elements in order of increasing ionization energy bismuth, lead, polonium, cesium Lowest Highest Please answer this question according to the general rules you have learned regarding periodic trends. DO NOT base your answer on tabulated values since exceptions may occur
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


