Question: 1. ( 2 points). Rank the following compounds, A-E, from lowest solubility in hexane, CH3(CH2)4CH3, to highest solubility in hexane. C D Your ranking: 2.
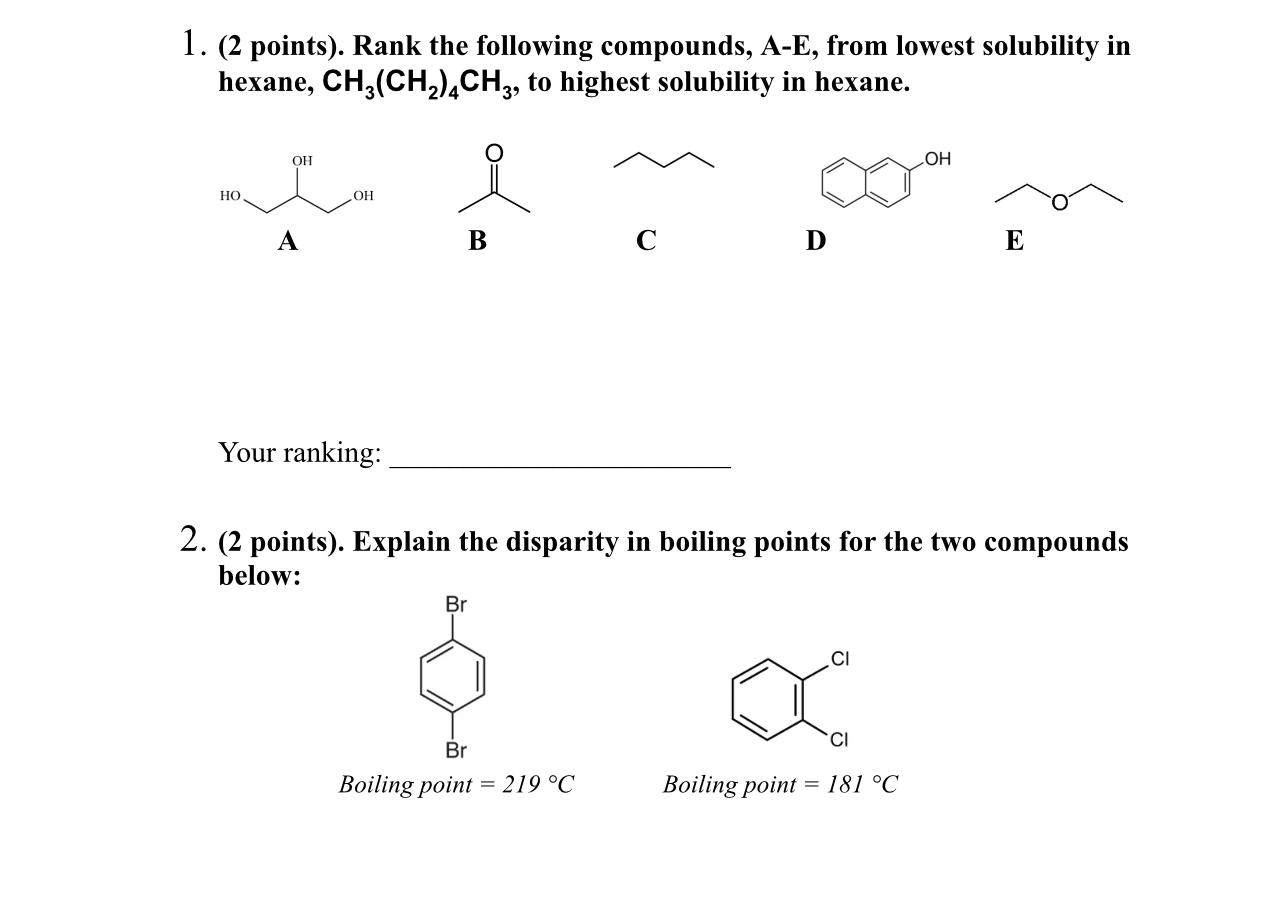
1. ( 2 points). Rank the following compounds, A-E, from lowest solubility in hexane, CH3(CH2)4CH3, to highest solubility in hexane. C D Your ranking: 2. ( 2 points). Explain the disparity in boiling points for the two compounds below
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


