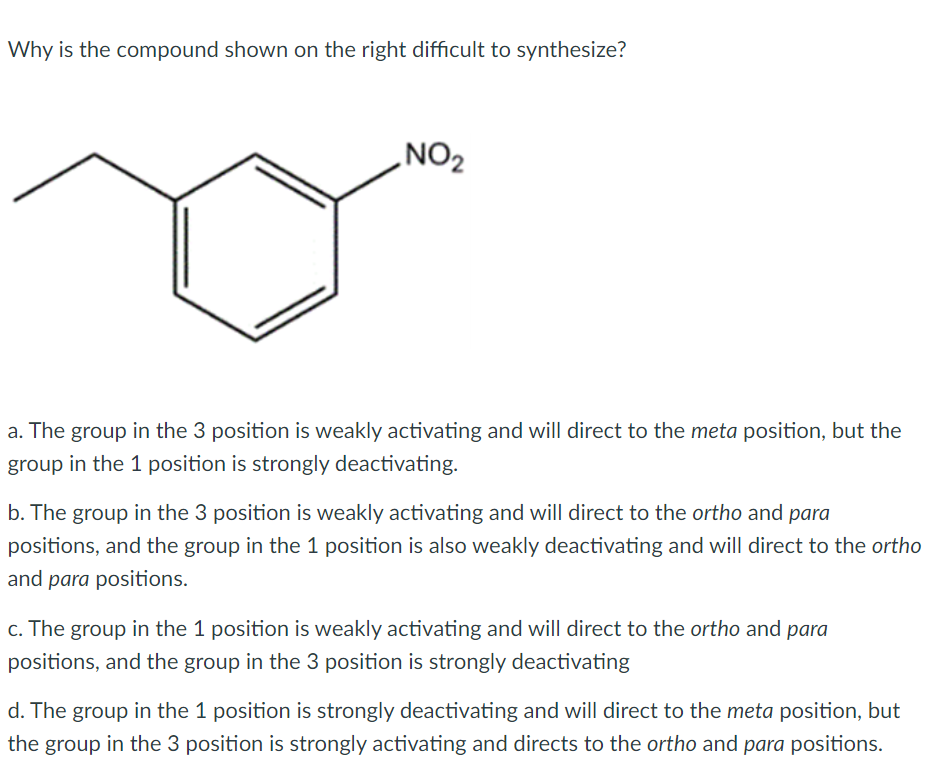
Question: 1. 2. Why is the compound shown on the right difficult to synthesize? NO2 a. The group in the 3 position is weakly activating and
1.
2.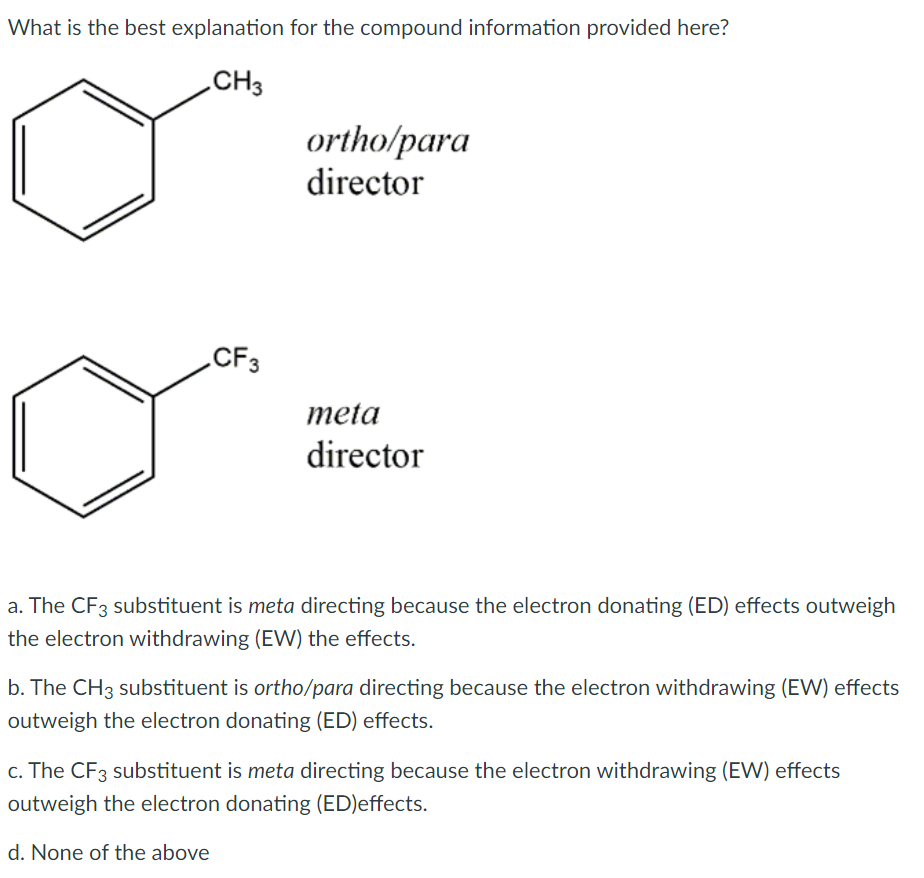
Why is the compound shown on the right difficult to synthesize? NO2 a. The group in the 3 position is weakly activating and will direct to the meta position, but the group in the 1 position is strongly deactivating. b. The group in the 3 position is weakly activating and will direct to the ortho and para positions, and the group in the 1 position is also weakly deactivating and will direct to the ortho and para positions. c. The group in the 1 position is weakly activating and will direct to the ortho and para positions, and the group in the 3 position is strongly deactivating d. The group in the 1 position is strongly deactivating and will direct to the meta position, but the group in the 3 position is strongly activating and directs to the ortho and para positions. What is the best explanation for the compound information provided here? CH3 ortho/para director CF3 meta director a. The CF3 substituent is meta directing because the electron donating (ED) effects outweigh the electron withdrawing (EW) the effects. b. The CH3 substituent is ortho/para directing because the electron withdrawing (EW) effects outweigh the electron donating (ED) effects. c. The CF3 substituent is meta directing because the electron withdrawing (EW) effects outweigh the electron donating (ED)effects. d. None of the above
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


